An Aluminum plasmonics-enriched ultrasensitive UV photodetector
Author(s)
Hsi-Sheng TengBiography
Prof. Teng is currently a chair professor of the Department of Chemical Engineering at National Cheng Kung University (NCKU) in Tainan, Taiwan. He is also jointly appointed to the Hierarchical Green Energy Materials (HiGEM) Research Center at NCKU as the associate director. His research specialties include Nanoporous Materials, Photovoltaic Solar Cells, Electrochemical Supercapacitors, Photoeletrodes for Water Splitting and Catalytic Reactions.
Academy/University/Organization
National Cheng Kung UniversitySource
1. Journal of Materials Chemistry A 2019, 7, 8384–8393. https://doi.org/10.1039/c8ta12123k
2. Journal of Materials Chemistry A 2016, 4, 2014-2048. http://dx.doi.org/10.1039/C5TA07780J
3. Advanced Materials 2014, Vol. 26, 3297-3303. http://dx.doi.org/10.1002/adma.201305299-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- ENGINEERING & TECHNOLOGIES
- Text & Image
- March 20,2020
In clean, renewable energy resource research, photocatalytic H2 production in an aqueous solution has received considerable attention. We have reported that water splitting over a semiconductor photocatalyst by using solar energy can produce H2 and O2 which has a high degree of sustainability, but the back reactions of the products on the catalyst surfaces result in inefficient photocatalytic H2 generation. Regarding the renewability of H2 resources, photocatalytic reforming of biomass materials, such as glucose (C6H12O6), sugar (sucrose, C12H22O11), and cellulose ((C6H10O5)n) represents a promising counterpart to photosynthesis for achieving a sustainable carbon cycle that produces clean solar fuels. Most studies have used TiO2 as the photocatalyst for reforming glucose or sugar to produce H2, but TiO2 has its limitation in solar light absorption. Carbon- or graphene-based photocatalysts, which are environmentally benign and sensitive to visible light, are considered as ideal media for producing clean and renewable H2. Performance of carbon- or graphene-based photocatalysts in the reforming of glucose, sugar, or cellulose for H2 production requires exploration. Our research efforts have proved that graphene dots are a promising catalyst for photoreforming of biomass materials into H2 in a practical manner; we achieved a H2-evolution apparent quantum yield of >20% under monochromatic irradiation at 420 nm.
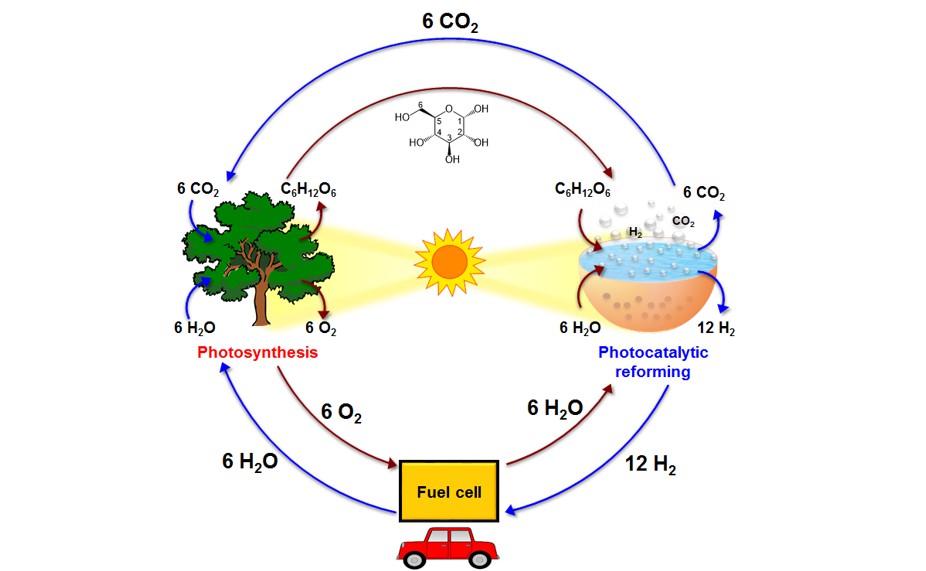
In clean, renewable energy resource research, photocatalytic H2 production in an aqueous solution has received considerable attention. Water splitting over a semiconductor photocatalyst by using solar energy to produce H2 and O2 has a high degree of sustainability, but the back reactions of the products on the catalyst surfaces result in inefficient photocatalytic H2 generation. Regarding the renewability of H2 resources, photocatalytic reforming of biomass materials, such as glucose (C6H12O6), sugar (sucrose, C12H22O11), and cellulose ((C6H10O5)n) represents a promising counterpart to photosynthesis for achieving a sustainable carbon cycle that produces clean solar fuels. Most studies have used TiO2 as the photocatalyst for reforming glucose or sugar to produce H2. Carbon- or graphene-based photocatalysts, which are environmentally benign and sensitive to visible light, are considered as ideal media for producing clean and renewable H2. Performance of carbon- or graphene-based photocatalysts in the reforming of glucose, sugar, or cellulose for H2 production requires exploration.
Graphene is a flat monolayer comprising sp2-bonded carbon atoms tightly packed into a two-dimensional honeycomb lattice. The lack of a band gap limits the applicability of graphene in photoenergy conversion, which requires semiconducting materials with a finite band gap. Graphene oxide (GO) is a form of graphene that has a band gap and contains various oxygen functional groups on the basal plane and sheet edge. The tunable electronic properties of graphene oxide (GO) nanomaterials make them interesting candidates for photoenergy conversion applications, including photoluminescence, photovoltaics and photocatalysis for water splitting.
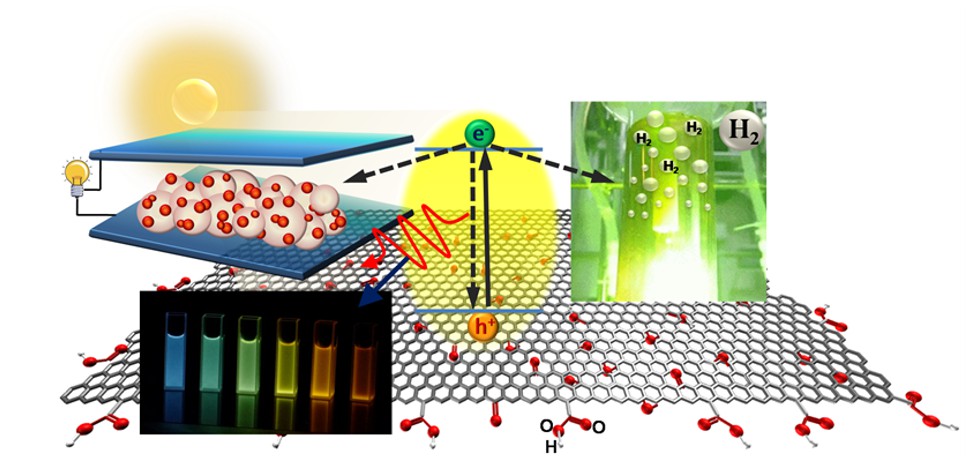
The oxygen functionalities on the graphene substrate enlarge the band gap and enable GO to easily disperse in an aqueous solution. Doping heteroatoms into GO matrices repairs the defects and modulates the electronic structure to increase their photocatalytic activity. We reported photocatalytic reforming of water into H2 and O2 over nitrogen-doped GO quantum dots (NGODs), which are based on the abundant elements C, H, O, and N. The NGODs exhibited both p- and n-type conductivities, with electronic properties similar to those of a p-n photochemical diode. The water-reforming mechanism was analogous to that of biological photosynthesis.
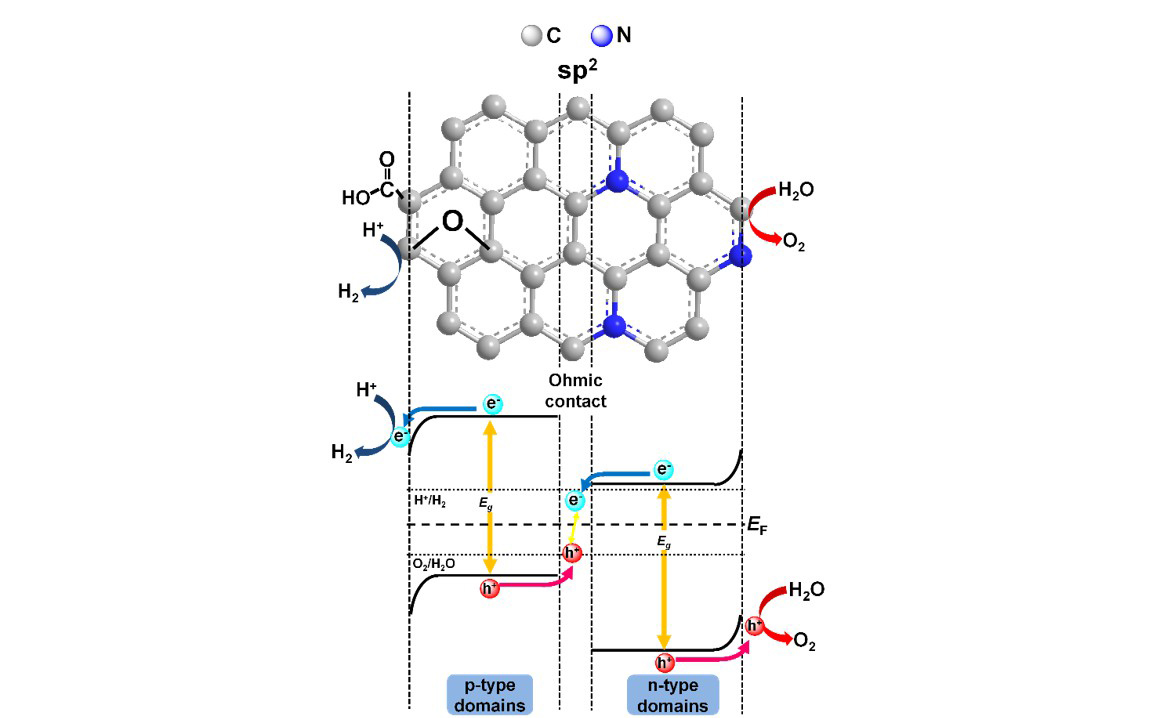
We reported that S and N can be introduced into the GO matrix to effectively repair the vacancy defects and facilitate the separation of photogenerated charges for interfacial reactions over the catalysts. S and N co-doped GO dots (SNGODs) exhibited a high content of graphitic N that was located inside the graphene sheets and repaired the vacancy defects of the basal plane. The peripheral N and S functionalities of the SNGODs produced electron resonance between the graphitic-π orbital and nonbonding states of the N and S atoms, resulting in the reduction of the band gap and the extension of the photogenerated charge lifetime. Pt-deposited SNGODs were stable in photocatalytic reforming of sugar and glucose into H2, with apparent quantum yields of 11% and 7.4%, respectively, under monochromatic irradiation at 420 nm. The high quantum yields in H2 production demonstrated the superiority of using graphene dots in the photocatalytic reforming of biomass into H2.
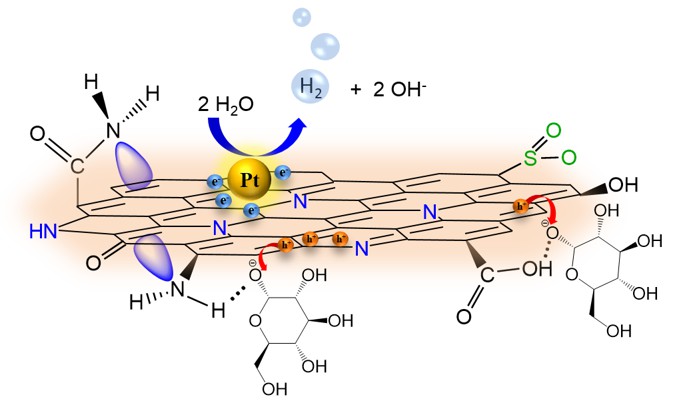
For practical applications, we have demonstrated the high efficiency of photocatalytic reforming of cellulose for H2 evolution over the SNGODs. Cellulose is the main constituent of biomass, a homopolymer consisting of glucose units, and is not completely soluble in aqueous solutions. With a pretreatment on cellulose, we achieved a H2-evolution apparent quantum yield of 23% under monochromatic irradiation at 420 nm.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.