Why a Good Innovation Ecosystem Matters

Author(s)
Bing-Joe HwangBiography
Professor Hwang is currently a Chair Professor in the Department of Chemical Engineering at National Taiwan University of Science and Technology (NTUST), director of the Sustainable Energy Development Center and director of the Building Energy Efficiency and Renewable Energy Center, Taiwan Building Technology Center. He has received the National Chair Professor and Academic Award from the Ministry of Education, the Outstanding Research Award from the Ministry of Science and Technology, the Academician of the Asian Pacific Academy of Materials and the Fellow of the International Society of Electrochemistry. Prof. Hwang has published more than 400 SCI papers and has more than 50 issued patents. His publications have received more than 20,000 citations, and he has an h-index of 71.
Academy/University/Organization
National Taiwan University of Science and TechnologySource
https://ntust518labfile.wixsite.com/nanoelectrochemistry-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- ENGINEERING & TECHNOLOGIES
- Text & Image; Video
- March 20,2020
Although the battery technology has made significant progress in recent years, the current performance of lithium ion batteries has not been able to meet the demands for high-energy and high-safety applications. At present, the energy density of lithium-ion batteries is about 200 Wh/kg or 600 Wh/L. Since the negative electrode has a certain volume or weight, the energy density of lithium ion batteries is limited. Although a thin-film lithium battery can be used to increase its energy density, the cost of its production is high and there are safety concerns in the production process. Prof. Hwang, at the Sustainable Energy Development Center, National Taiwan University of Science and Technology, has long been developing advanced materials for novel batteries and fuel cells. Recently, together with his group members, Prof. Hwang has aimed to develop anode-free lithium batteries through the engineering of electrolytes and artificial solid electrolyte interphase (a-SEI). During the charging process of an anode-free battery, lithium ions leave the positive electrode and are deposited in the form of lithium metal on the negative current collector, and the device naturally becomes a thin-film lithium battery. With this unique configuration design, this battery can provide an ultra-high energy density of about 400 Wh/kg or 1200 Wh/L.
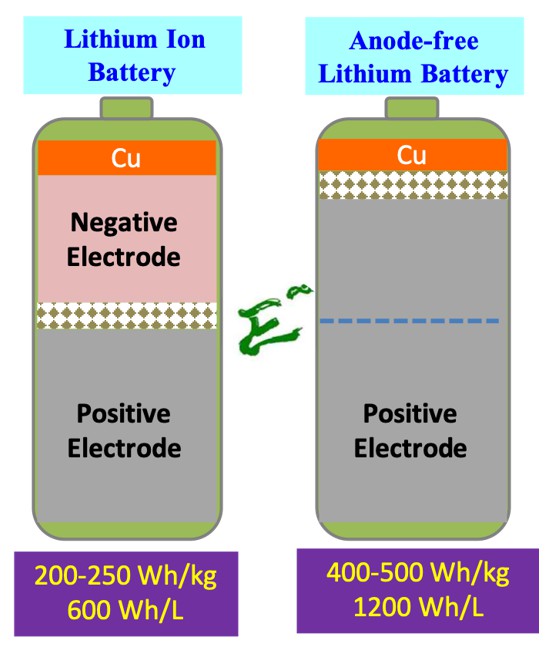
The energy density of the current commercial lithium-ion batteries is about 200 Wh/kg or 600 Wh/L. The energy density of an anode-free lithium battery is about 2-3 times the current commercial lithium-ion batteries. Meanwhile, its production process is not only simple but low-cost. However, its charging and discharging process involves the phenomena of lithium metal deposition and dissolution. Because of the highly reactive nature of lithium metal, the electrolyte decomposition and the formation of dead lithium cause the continuous loss of active lithium with an increased number of cycles. Therefore, the capacity of the anode-free lithium battery will fade accordingly. In order to overcome these challenging issues, Prof. Hwang's team aims to improve the performance of anode-free lithium batteries through the engineering of electrolytes and artificial solid electrolyte interphase (a-SEI). This article will briefly introduce how to improve the performance of anode-free lithium batteries by electrolyte engineering.
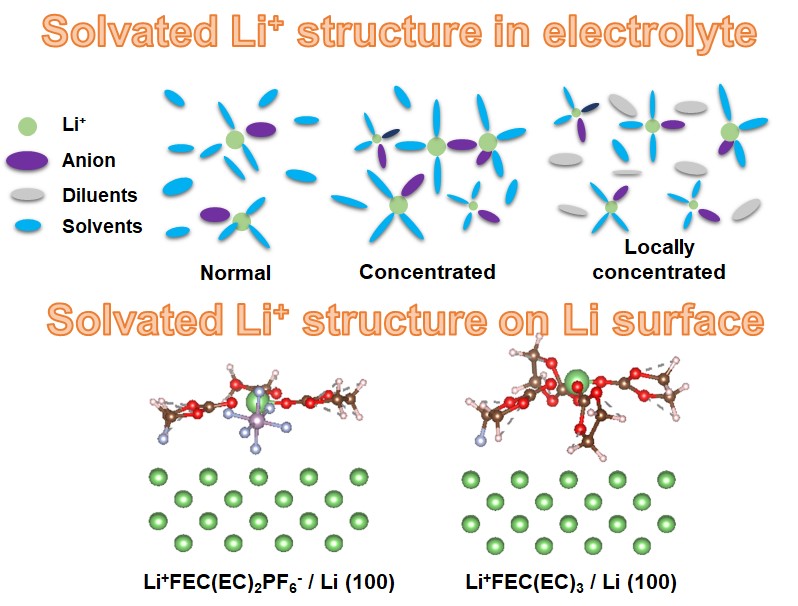
First of all, Prof. Hwang's team used the concept of locally concentrated electrolytes. The difference between high concentrated electrolytes and general electrolytes is the change in the solvation structure of lithium ions. In general electrolytes, lithium ions are mainly solvated by solvents with strong solvation capability, such as ethylene carbonate (EC). In highly concentrated electrolytes, the number of solvents solvated with lithium ions will decrease and ion pairs will form. This not only changes the solvation structure of lithium ions in the electrolyte, but, more importantly, it is understood from the team's theoretical calculations that adsorbed species on the lithium surface will change from a diluted electrolyte to a highly concentrated one. The result can further regulate the composition of the solid electrolyte interphases (SEI), and obtain an excellent SEI, which can inhibit the formation of lithium dendrites and improve the Coulombic efficiency. However, the disadvantages of highly concentrated electrolytes are their high cost and low ionic conductivity due to the high viscosity. Prof. Hwang’s team used a solvent with a lower solvation capacity as a diluent to dilute it. In addition to obtaining comparable ionic conductivity with commercial electrolytes by reducing its viscosity, its local solvation structure and derived SEI can be maintained as in highly concentrated electrolytes, as shown in the figure below. Prof. Hwang's team has developed an electrolyte with excellent performance, which can greatly improve the performance of anode-free lithium batteries.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




