Dyslipidemia Is the Seed of Arrhythmia-related Aging Disabilities
Author(s)
Yuan Soon HoBiography
Dr. Ho is currently a distinguished professor in the School of Biomedical Technology, Taipei Medical University and is the Associate Director of the TMU Research Center of Cancer Translational Medicine.
Academy/University/Organization
Taipei Medical University-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- September 25,2019
While smoking is a well-known risk factor for cancer, non-nicotine components of tobacco have generally been thought to be the carcinogens, so little is known about how nicotine acts on cells to promote cancer cell growth. To determine whether nicotine works on the cellular level to promote breast cancer growth, we looked at 276 breast tumor samples from anonymous donors to the Taipei Medical University Hospital, to see whether subunits of the nicotinic acetylcholine receptor were overexpressed in breast cancer cells compared with the surrounding normal cells. The results indicated that the α9-nicotinic acetylcholine receptor (α9-nAChR) is a molecular marker for smoking-induced breast cancer formation. In addition, we found that ~90 % of α9-nAChR was expressed in monocytes isolated from Taiwanese volunteers (n=55 per group), and such results clearly indicate that α9-nAChR is important for smoking-induced foam cells (which are the pre-lesion of plaque formation). The smokers’ monocytes that expressed high levels of α9-nAChRs tended to engulf more oxidized low-density lipoprotein (ox-LDL) to become lipid-laden macrophages (foam cells). These results imply that receptor-mediated signals play a decisive role in biological functions related to human chronic diseases. Identification of natural compounds from food or fruits effectively prevent smoking-induced diseases. In addition, development of therapeutic drugs targeting the α9-nAChR should have potential for further clinical applications.
For breast cancer in particular, some large epidemiological studies have shown that smoking is related to increased breast cancer risk, but they have not been accompanied by molecular biology studies on how that actually works. Our previous study (J Natl Cancer Inst, 102(17):1322-1335, 2010) demonstrated that the expression levels of α9-nAChR in breast cancer tissues were very high (>8-fold higher than the level in normal tissue) in Taiwanese patients (n=276), particularly in the tissues of late-stage patients (stages 3–4). We also demonstrated that extremely low concentration nicotine (less than 10 nM) specifically binds to the α9-nAChR within 60 minutes, which should be important to promote smoking addiction and may also directly promote the development of breast cancer. Besides, we found that reducing the levels of α9-nAchRs inhibited tumor growth in laboratory experiments, whereas increasing the levels of α9-nAchRs or treating more normal breast cells with nicotine promoted the development of early stage cancer characteristics. All these data implied that the α9-nAChR is attractive for the development of novel therapeutic agents.
We observed that women with breast tumors with high α9-nAChR mRNA expression levels had the lowest 5-year disease-specific survival rate. The study suggests that not only could smoking be causally related to breast carcinogenesis but also that nicotine could directly contribute to the molecular mechanism of carcinogenesis in addition to indirectly contributing by promoting addiction to smoking.
To test the hypothesis, an epidemiological cohort study was performed to assess the clinical significance of α9-nAChR expression in breast tumors of different stages and to correlate α9-nAChR expression with smoking history among Taiwanese women. The results revealed the increased expression of α9-nAChR mRNA in tumor tissues from current smokers relative to those from passive smokers and nonsmokers (6.62-fold vs. 2.8-fold or 1.51-fold, respectively). Large epidemiological cohort studies in the United States have indicated that active and passive smoking are associated with increased breast cancer risk. However, there was no direct evidence of an effect of tobacco carcinogens on the cellular molecules involved in breast tumorigenesis. These results provide molecular evidence for smoking-induced breast cancer formation and suggest that alterations in some membrane proteins and the pathways they regulate have been established as cancer hallmarks for carcinogenesis, cancer migration and treatment resistance.
Based on the α9-AChR study in breast cancer, we recently (Nature Communication 2019,10:3131, https://www.nature.com/articles/s41467-019-10920-8) presented a systematically integrated method to generate a resource of cancer membrane protein-regulated networks (http://campnets.life.nctu.edu.tw). We identified α9-nAChR, with 13 new protein-protein interactions (e.g., Her-2), as a potential therapeutic target and found a novel antimetastasis agent, bupropion, for treatment in nicotine-induced breast cancer. According to our previous study, targeting on the membrane α9-nAChR in breast cancer cells significantly reduced the tumor growth formation in breast cancer animal models (J Natl Cancer Inst, 102(17):1322-1335, 2010). Such results support the hypothesis that developing therapeutic antibodies specific to the α9-nAChR should be useful for breast cancer patients.
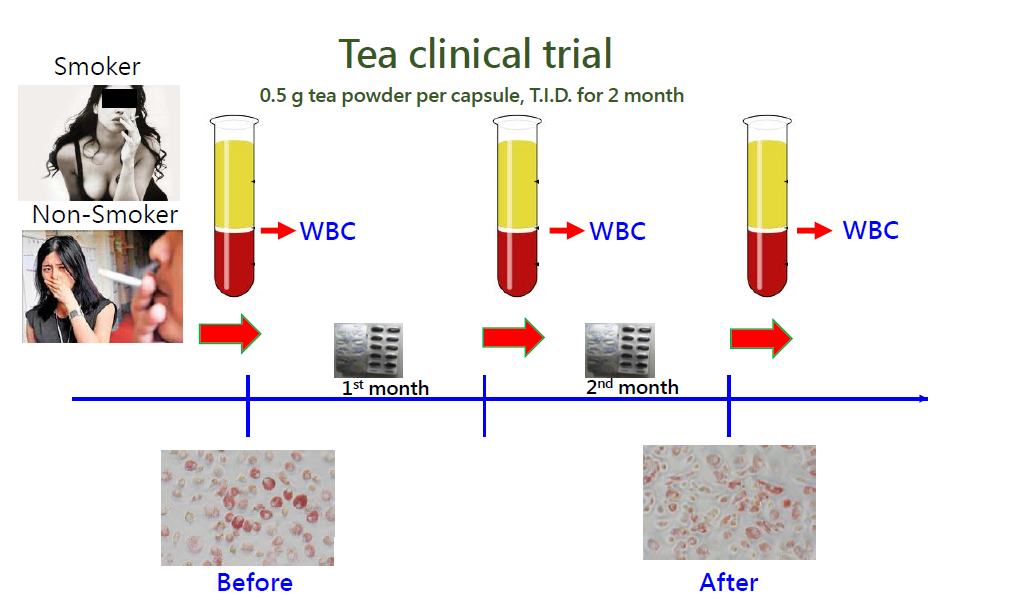
For this purpose, several previous studies from our group demonstrated that α9-nAChR inhibitors isolated from natural products, such as garcinol (from G. indica dried fruit rind), EGCG (from tea leaves), and luteolin and quercetin (from fruits and vegetables), could be used as chemopreventive agents for smoking-induced breast cancer. To assess whether drinking tea can effectively prevent smoking-induced atherosclerosis, an ex vivo clinical trial was performed. The volunteers were randomly allocated into two groups (n=55 per group) that received either Pu-erh tea powder or a cellulose placebo (0.5 g/capsule, T.I.D.) for two months. We found that monocytes displaying high α9-nAChR expression isolated from heavy smokers before consuming tea capsules tended to form foam cells in response to nicotine. In contrast, long-term tea consumption significantly downregulated monocytic α9-nAChR expression, which caused circulating monocytes to resist nicotine-induced foam cell formation even in heavy smokers. This study suggests that tea drinking effectively attenuates the initial step (foam cell formation) of smoking-induced atherosclerosis in circulating monocytes.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




