Towards Smarter Medicine and Healthcare with AI

Author(s)
Yow-Ling ShiueBiography
Prof. Yow-Ling Shiue is currently a Professor/Chair of the Institute of Biomedical Sciences, National Sun Yat-sen University. Her team has recently focused on the biological function and clinical significance of novel membrane proteins in urinary bladder urothelial carcinomas, since membrane proteins are major drug targets.
Academy/University/Organization
National Sun Yat-sen UniversitySource
https://clincancerres.aacrjournals.org/content/23/24/7650.long-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- September 23,2019
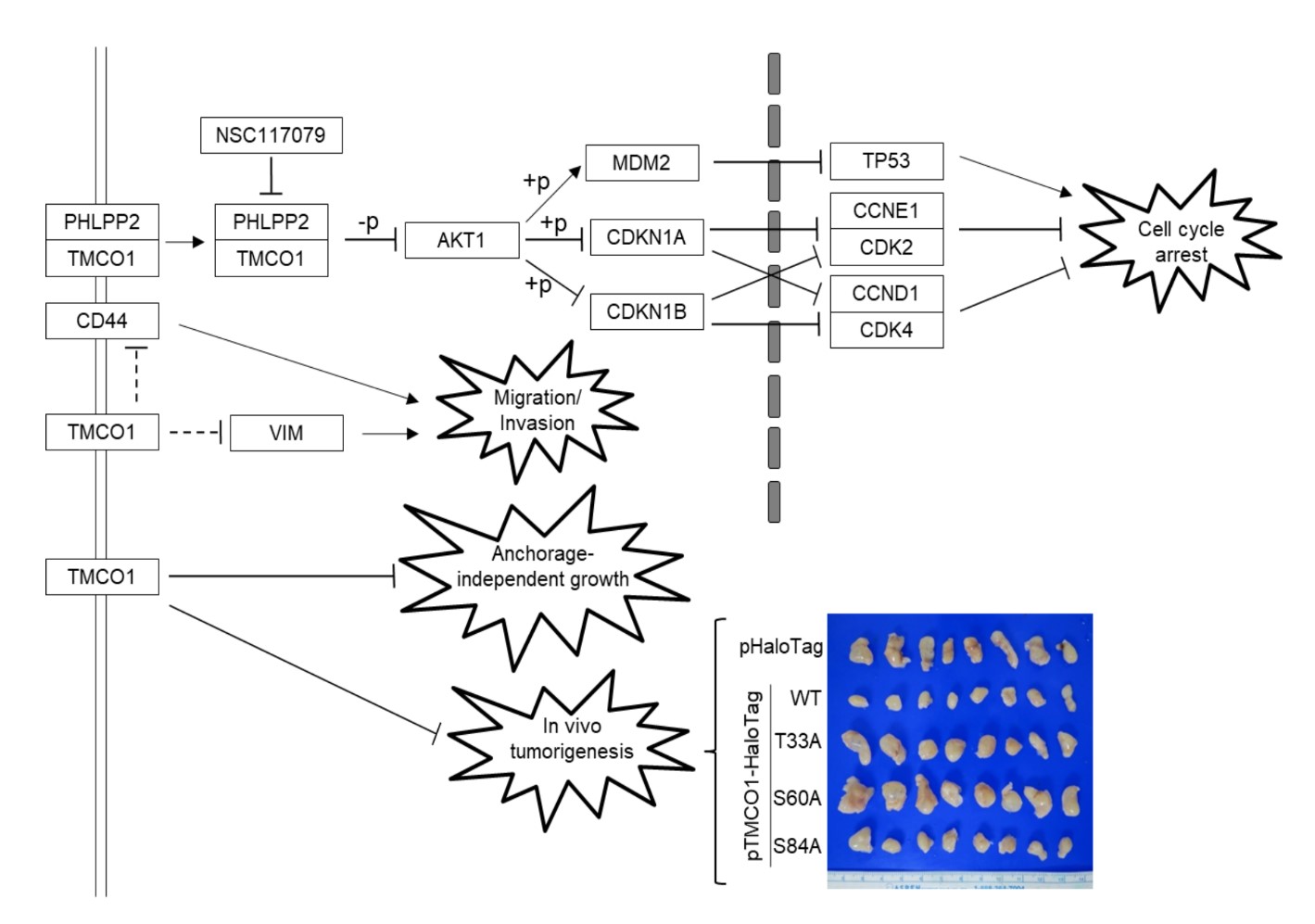
Urinary bladder urothelial carcinoma (UBUC) is a common malignant disease in developed countries. Cell-cycle dysregulation resulting in uncontrolled cell proliferation has been associated with UBUC development. This study aimed to explore the roles of TMCO1 in UBUCs. Data mining, branched DNA assay, immunohistochemistry, xenograft, cell culture, quantitative RT PCR, immunoblotting, stable and transient transfection, lentivirus production and stable knockdown, cell-cycle, cell viability and proliferation, soft-agar, wound-healing, transwell migration and invasion, coimmunoprecipitation, immunocytochemistry, and AKT serine/threonine kinase (AKT) activity assays and site-directed mutagenesis were used to study TMCO1 involvement in vivo and in vitro. Data mining identified that the TMCO1 transcript was downregulated during the progression of UBUCs. In distinct UBUC-derived cell lines, changes in TMCO1 levels altered the cell-cycle distribution, cell viability, cell proliferation, and colony formation, and modulated the AKT pathway. TMCO1 recruited the PH domain and leucine-rich repeat protein phosphatase 2 (PHLPP2) to dephosphorylate pAKT1(serine 473). Mutagenesis at S60 of the TMCO1 protein released TMCO1-induced cell-cycle arrest and restored the AKT pathway in BFTC905 cells. Stable TMCO1 (wild-type) overexpression suppressed, whereas T33A and S60A mutants recovered, tumor size in xenograft mice. Clinical associations, xenograft mice, and in vitro indications provide solid evidence that the TMCO1 gene is a novel tumor suppressor in UBUCs. TMCO1 dysregulates cell-cycle progression via suppression of the AKT pathway, and S60 of the TMCO1 protein is crucial for its tumor-suppressor roles.
.jpg)
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.