A New Direction for Neuro-Rehabilitation Therapy in Stroke

Author(s)
Han-Chung WuBiography
Prof. Wu is a Distinguished Research Fellow at ICOB, and the Director of the BioTReC, Academia Sinica. His research mainly focuses on infectious diseases and cancer. He holds 97 patents, and has published more than 110 articles, as well as receiving 25 honors and awards, including the Fellow of the National Academy of Inventors.
Academy/University/Organization
Academia SinicaSource
https://cancerres.aacrjournals.org/content/80/22/5035.short
https://www.sciencedirect.com/science/article/pii/S0304383518304488-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- July 13,2021
A recent study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, has greatly advanced our knowledge of how EpCAM acts to promote cancer cell growth and how that process can be stopped. It is reported that EpCAM overexpressing on malignant cells promotes cancer progression and can be an excellent target for cancer treatment. In this study, the EpCAM-neutralizing antibody, EpAb2-6, developed by Wu’s research group was used to inhibit the function of EpCAM and directly induced EpCAM-positive cancer cells apoptosis. More importantly, EpCAM blockade could decrease PD-L1 protein stability and enhance the cytotoxic activity of CD8+ T cells. The combination of EpAb2-6 with Atezolizumab, an anti-PD-L1 antibody, almost completely eliminated tumors. Moreover, the number of CD8+ T cells in combination-treated tumors was increased compared to Atezolizumab alone. Therefore, the recent findings suggest an exciting new combined treatment strategy for cancer immunotherapy in patients with EpCAM-expressing tumors.
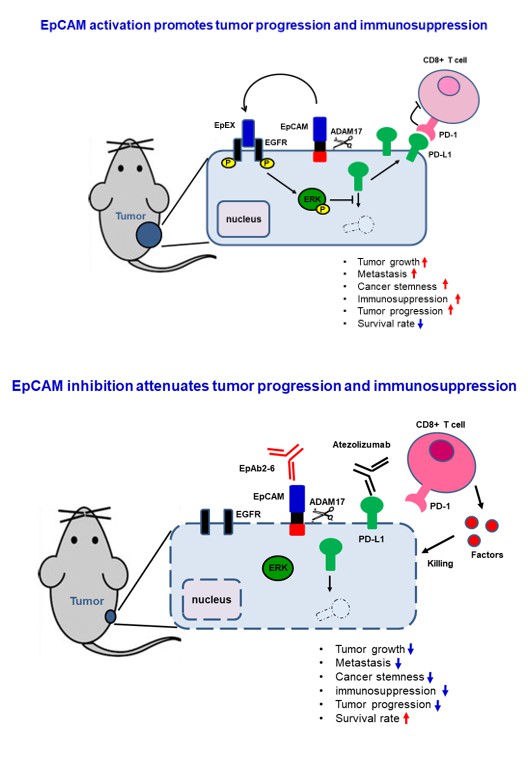
Figure 1. The cell-surface molecule EpCAM
In the world of cutting-edge cancer research, the cell-surface molecule EpCAM is widely thought of as a promising target for new cancer diagnostics and therapeutics. A recent study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, has greatly advanced our knowledge of how EpCAM acts to promote cancer cell growth and how that process can be stopped. The study was published in the world-renowned journal, Cancer Research. In this study, Dr. Wu’s group reported that the EpCAM extracellular domain (EpEX) binds EGFR, activating both AKT and MAPK signaling to respectively inhibit FOXO3a function and stabilize PD-L1 protein. Treatment with the EpCAM-neutralizing antibody, EpAb2-6, developed by Wu’s research group, could inhibit AKT and FOXO3a phosphorylation, increase FOXO3a nuclear translocation, and upregulate HtrA2 expression to promote apoptosis, while decreasing PD-L1 protein levels to enhance the cytotoxic activity of CD8+ T cells. Other methods for decreasing PD-L1 action have proven quite effective in the clinic. The use of immune checkpoint inhibitors, such as monoclonal antibodies against CTLA-4, PD-1 and PD-L1, represents a major breakthrough in cancer treatment. These therapeutics can greatly extend the life of cancer patients and make many previously incurable cancers curable. Based on this clinical success, immune checkpoint inhibitors like CTLA4 and PD-1 earned the Nobel Prize in Physiology or Medicine in 2018.
Since EpCAM is enriched in many types of cancer cells, the findings and tools from this study have the potential to broadly impact cancer diagnosis and treatment. In vivo, EpAb2-6 markedly extended survival in mouse metastasis and orthotopic models of human colorectal cancer (Figure 1). The combination of EpAb2-6 with Atezolizumab, an anti-PD-L1 antibody, almost completely eliminated tumors. Moreover, the number of CD8+ T cells in combination-treated tumors was increased compared to Atezolizumab alone. Therefore, the recent findings suggest an exciting new combined treatment strategy for cancer immunotherapy in patients with EpCAM-expressing tumors.
EpAb2-6 is currently the only antibody in the world that directly induces apoptosis to the cancer cells. In this context, previously published anti-EpCAM antibodies such as MT201 and ING-1 were shown to exhibit their therapeutic effect via ADCC/CDC in clinical trials. However, EpAb2-6 was shown to exhibit its mechanism of action (MoA) by directly inducing apoptosis along with ADCC/CDC, thus prolonging the overall survival of animal models of pancreatic and colorectal cancer. Hence, the MoA, complementarity determining sequence (CDR) and the epitope of EpAb2-6 are new inventions. On this basis, we obtained patents in the USA, Japan, the EU, and China. Most importantly, more than 85% of cancer types highly express EpCAM for which EpAb2-6 might be a potential therapeutic strategy for various cancer treatments. Since there is currently no anti-EpCAM antibody drug on the global market, our antibody may have impactful essentiality.
EpAb2-6 is patent-protected in many countries around the world. Recently, the group obtained NT$50 million funding from the TRUST-U (Taiwan Reputed University Start-ups to Taiwan Unicorns) program to be used for therapeutic antibody drug development. With this funding, they further established a biomedical start-up company at the National Biotechnology Research Park that can bridge the gap between basic science and clinical application.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.