Development of an Anti-EpCAM Antibody for the Treatment of Colorectal Cancer

Author(s)
Yen-Hua HuangBiography
Dr. Yen-Hua Huang is currently the Distinguished Professor and Dean of the Office of Research and Development, Taipei Medical University, and the CEO of TMU's Research Center for Cell Therapy and Regeneration Medicine. Prof. Huang has an established outstanding track record in stem cell biology, pathology, and cell therapy, particularly in embryonic pluripotency regulation, systemic safety cell therapy product development, and cancer stemness.
Academy/University/Organization
Taipei Medical University-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- September 25,2019
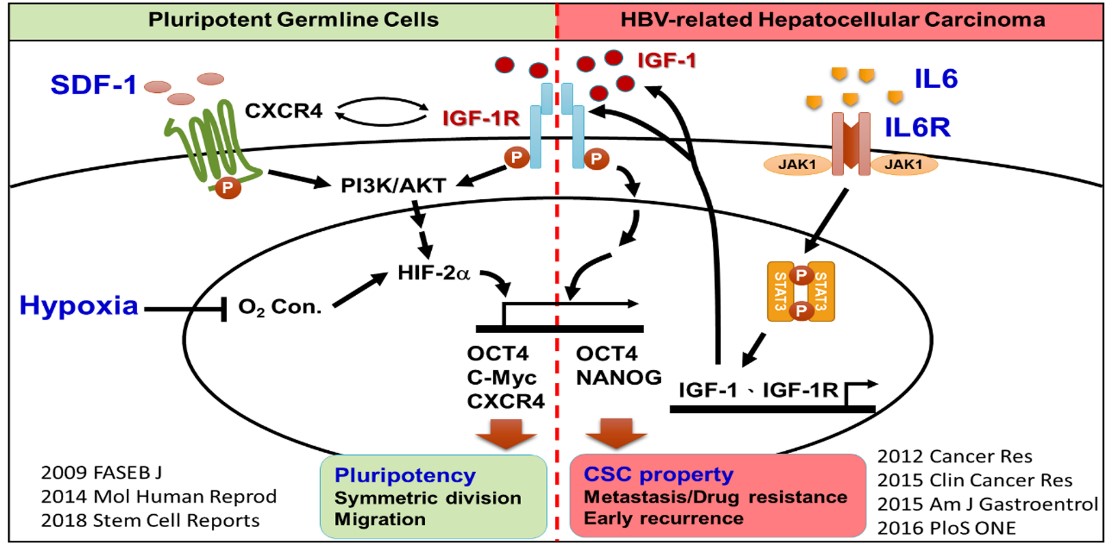
Hypoxia cooperates with endocrine signaling to maintain the symmetric self-renewal proliferation and migration of embryonic germline stem cells (GSCs). However, the lack of an appropriate in vitro cell model has dramatically hindered the understanding of the mechanism underlying this cooperation. We have previously established a novel serum-free culture system to generate mouse pluripotent primordial germ cell-like (PGC-like) stem cells in vitro. Using this unique serum-free culture condition, we demonstrate how niche IGF-1R signaling plays a critical role in maintaining OCT4 expression and germ cell pluripotency. Further using an in vitro hypoxic niche condition, we demonstrate that the critical role of the embryonic hypoxic niche is able to cross-interact with the IGF-1/IGF-1R and SDF-1/CXCR4 signaling to regulate the symmetrical division and migration of pluripotent germline stem cells. In addition, we also find that the niche inflammatory cytokine IL-6 induces the expression of IGF-1/IGF-1R/OCT4 for driving the initiation of stemness-related properties and the early recurrence and poor prognosis in patients with the HBV-related hepatocellular carcinomas (HBV-HCC). Taken together, we demonstrate the bi-functional niche IGF-1R signaling in pluripotency regulation of embryonic germline stem cells and cancer reprogramming, in particular for the interplay between niche microenvironments and the IGF-1R signaling. These findings greatly extend the current understanding from single point of tumor microenvironment to learn from the embryonic niche and signaling. The IGF-1R will respond to its unique niche environment to play a critical role in OCT4 expression to drive stem cell symmetric division, migration, and somatic cancer cell reprogramming.
Niche environments are known to play critical roles in stem cell biology and pathology. The expression of pluripotency-related genes, such as the transcriptional factor OCT4, is highly associated with both embryo development and tumor drug susceptibility. Additionally, when working on the bedside stem cell therapy, the in vitro culture niche would absolutely control the stem cell potency. Currently, the interaction between the niche and stemness expression still remains unclear. My research interests are focused on the niche-stem cell dialogues, including embryonic pluripotency, cancer reprogramming, and stem cell potency. We especially pay attention to two major effects which are highly associated with stemness expression in pluripotent embryonic germline stem cells and cancer cell reprogramming: niche hypoxia and niche inflammation.
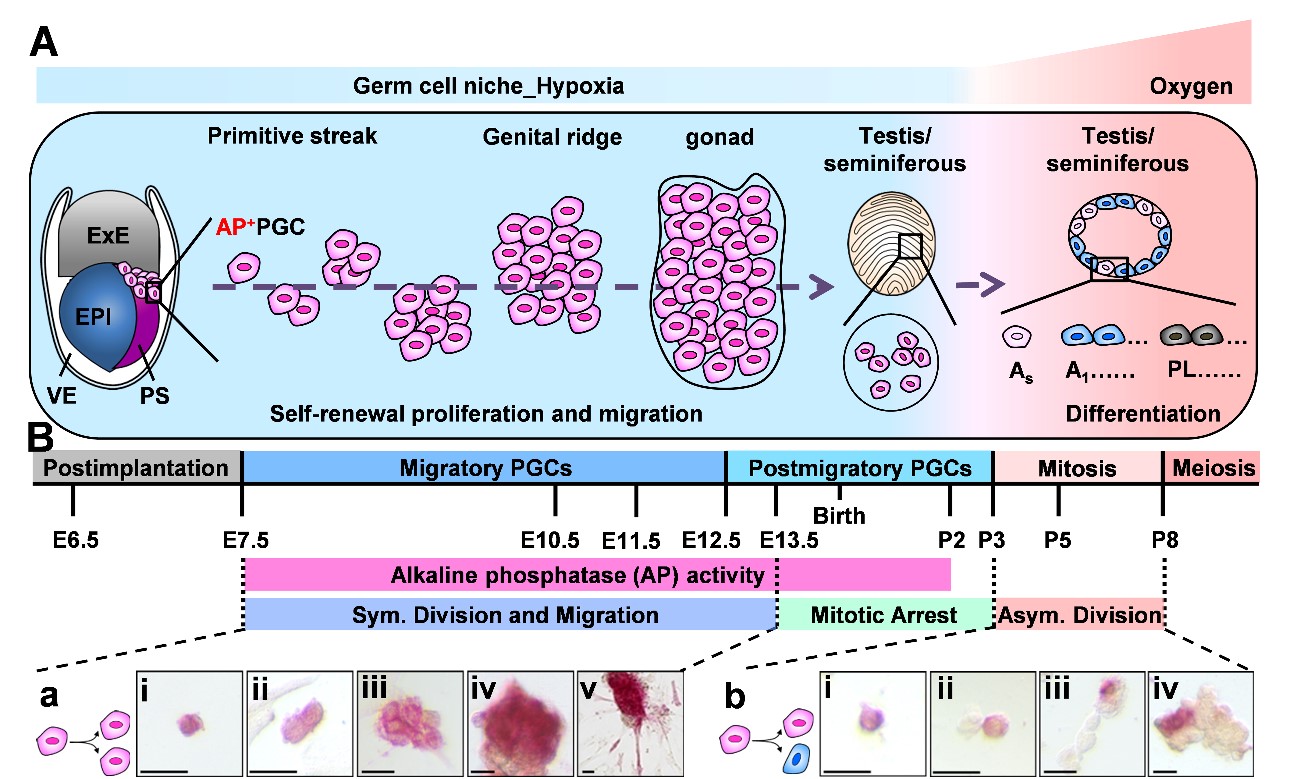
In the aspect of the embryonic hypoxia niche in stem cell pluripotency, we are interested in the niche interplay of pluripotency regulation, and particularly in learning from the nature embryonic stem biology, and extending to study how the tumor microenvironment hijacks the embryonic signal to support their cancer reprogramming in stem pathology. We have demonstrated that hypoxia cooperates with endocrine signaling to maintain the pluripotency of embryonic germline stem cells (GSCs). However, the lack of an appropriate in vitro cell model has dramatically hindered the understanding of the mechanism underlying this cooperation. The general culture condition with undefined serum components in certain serum batches dramatically limits the germ cell ability to acquire stem cell pluripotency. In this aspect, we have established a unique in vitro serum-free culture platform to cultivate mouse pluripotent primordial germ cell-like (PGC-like) germline stem cells (GSCs). With the unique serum-free culture condition, we successfully generated mouse alkaline phosphatase positive (AP+) GSCs. Hypoxia has been known to stabilize HIF proteins that increase the expression levels HIF-2α/IGF-1/IGF-1R/OCT4, particularly for HIF-2α binding to the hypoxia-response elements of the OCT4 promoter region, which then initiates OCT4 expression in cells encountering O2 deprivation conditions. Therefore, we found that niche hypoxia would greatly increase the cell proliferation and the levels of nuclear OCT-4/HIF-2α protein of AP+GSCs. By using in vitro and in vivo study models combined with RNA interference genetic editing, we demonstrated the mechanism of niche hypoxia cooperating with the autocrinal/paracrinal IGF-1/IGF-1R–PI3K/Akt–mTOR–HIF-2α–OCT4 signal loop in maintaining embryonic stem cell pluripotency. Findings in these works provide insights into the niche endocrinology in underlying early germ cell development [1,2]. Furthermore, we demonstrated that hypoxia significantly induced both the symmetric self-renewal proliferation and mesenchymal transition of AP+GSC, and increased the expression levels of the pluripotent transcription factor OCT4 and migration-associated proteins (SDF-1, CXCR4, IGF-1, and IGF-1R), and activated the cellular expression and translocalization of the CXCR4-downstream proteins ARP3/pFAK. The underlying mechanism involved significant IGF-1/IGF-1R activation of OCT4/CXCR4 expression through HIF-2α regulation. Picropodophyllin-induced inhibition of IGF-1R phosphorylation significantly suppressed hypoxia-induced SDF-1/CXCR4 expression and cell migration. Furthermore, transactivation between IGF-1R and CXCR4 was involved [3]. Findings from these works demonstrated that niche hypoxia synergistically cooperates with its associated IGF-1R signaling to regulate the symmetric division (self-renewal proliferation) and cell migration of AP+GSCs through HIF-2α–OCT4/CXCR4 during embryogenesis.
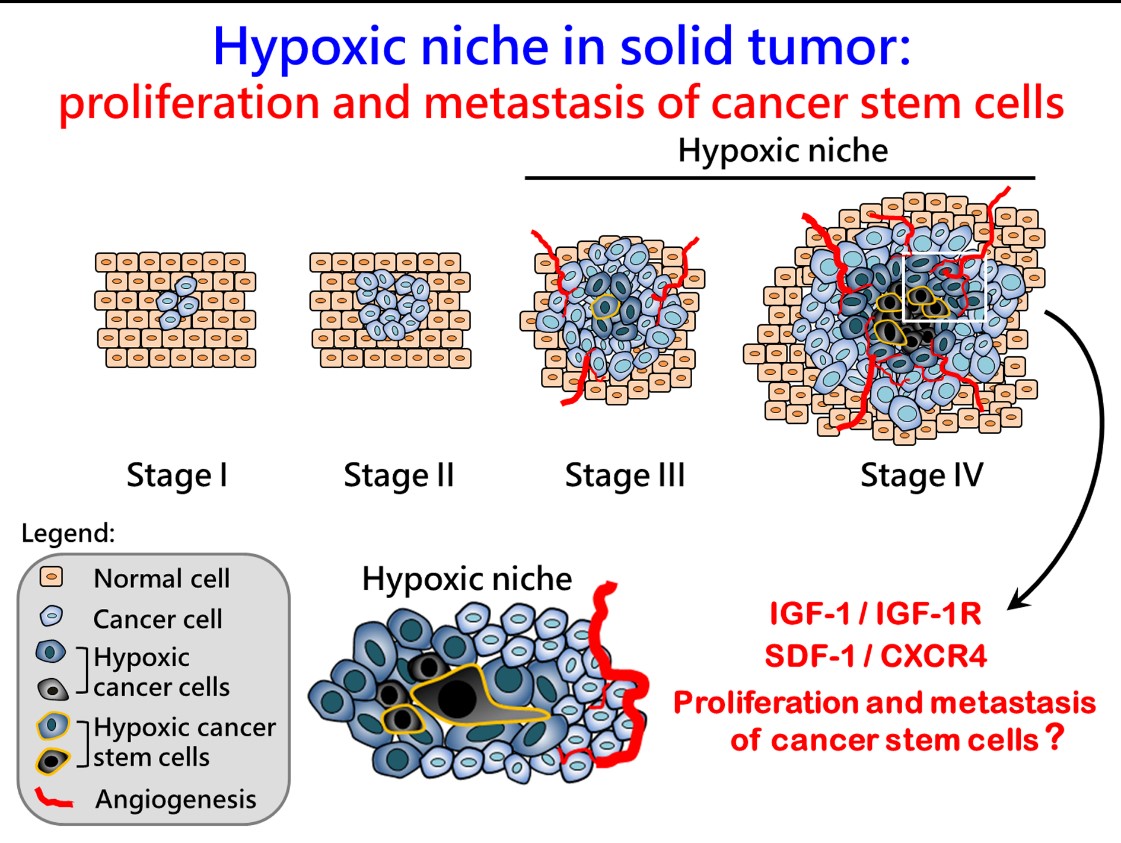
When working on the cancer biology, we found that a similar hypoxic niche exists in late stage solid tumors which generally share some similar characteristics with embryonic stem cells, and importantly, the inflammation niche is highly associated with tumor progression. For example, tumorigenic niche hypoxia can lead to high expression levels of IGF-1 and IGF-1R, and may cause both OCT4 stemness-related protein and SDF-1/CXCR4 migration-related proteins expression, further resulting in cancer cell reprogramming. The HBV-related hepatocellular carcinomas (HBV-HCC) is a perfect disease model to study the effect of niche inflammation on cancer reprogramming: (1) HCC is the major disease in Asia, including Taiwan. We can have enough patient tumor tissue to verify the clinical impacts. (2) The liver chronic inflammation caused by HBV-infection has been well documented. (3) The HBV virus infection may regulate the epigenetic factors which directly affect the expressions of stemness-related genes such as the OCT4.
- Huang, Y.H.; Chin, C.C.; Ho, H.N.; Chou, C.K.; Shen, C.N.; Kuo, H.C.; Wu, T.J.; Wu, Y.C.; Hung, Y.C.; Chang, C.C., et al. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1- dependent pathway. FASEB J 2009, 23, 2076-2087.
- Huang, Y.H.; Lin, M.H.; Wang, P.C.; Wu, Y.C.; Chiang, H.L.; Wang, Y.L.; Chang, J.H.; Huang, Y.K.; Gu, S.Y.; Ho, H.N., et al. Hypoxia inducible factor 2alpha/insulin-like growth factor receptor signal loop supports the proliferation and Oct-4 maintenance of mouse germline stem cells. Mol Hum Reprod 2014, 20, 526-537.
- Kuo, Y.C.; Au, H.K.; Hsu, J.L.; Wang, H.F.; Lee, C.J.; Peng, S.W.; Lai, S.C.; Wu, Y.C.; Ho, H.N.; Huang, Y.H. IGF-1R Promotes Symmetric Self-Renewal and Migration of Alkaline Phosphatase(+) Germ Stem Cells through HIF-2alpha-OCT4/CXCR4 Loop under Hypoxia. Stem Cell Reports 2018, 10, 524-537.
- Chang, T.S.; Chen, C.L.; Wu, Y.C.; Liu, J.J.; Kuo, Y.C.; Lee, K.F.; Lin, S.Y.; Lin, S.E.; Tung, S.Y.; Kuo, L.M., et al. Inflammation Promotes Expression of Stemness-Related Properties in HBV-Related Hepatocellular Carcinoma. PLoS One 2016, 11, e0149897.
- Chang, T.S.; Wu, Y.C.; Chi, C.C.; Su, W.C.; Chang, P.J.; Lee, K.F.; Tung, T.H.; Wang, J.; Liu, J.J.; Tung, S.Y., et al. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin Cancer Res 2015, 21, 201-210.
- Au, H.K.; Chang, J.H.; Wu, Y.C.; Kuo, Y.C.; Chen, Y.H.; Lee, W.C.; Chang, T.S.; Lan, P.C.; Kuo, H.C.; Lee, K.L., et al. TGF-betaI Regulates Cell Migration through Pluripotent Transcription Factor OCT4 in Endometriosis. PLoS One 2015, 10, e0145256.
- Chang, T.S.; Wu, Y.C.; Tung, S.Y.; Wei, K.L.; Hsieh, Y.Y.; Huang, H.C.; Chen, W.M.; Shen, C.H.; Lu, C.H.; Wu, C.S., et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Am J Gastroenterol 2015, 110, 836-844; quiz 845.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.