EditCell Virology Platform to Develop Broad-Spectrum Antivirals against COVID-19

Author(s)
Hsiang-Chun LeeBiography
Dr. Lee is currently Assistant Professor, Director, Lipid Science and Aging Research Center (LSARC) in Kaohsiung Medical University (KMU), and a Cardiologist in KMU hospital. Dr. Lee’s PhD thesis presents novel arrhythmia mechanisms. Her research overarching goal has been aimed at the lipotoxicity of VLDL on heart and vascular aging.
Academy/University/Organization
Kaohsiung Medical UniversityEdited by
Hsiang-Chun LeeSource
https://www.mdpi.com/1422-0067/17/1/134
https://www.mdpi.com/2077-0383/8/6/881-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- August 21,2019
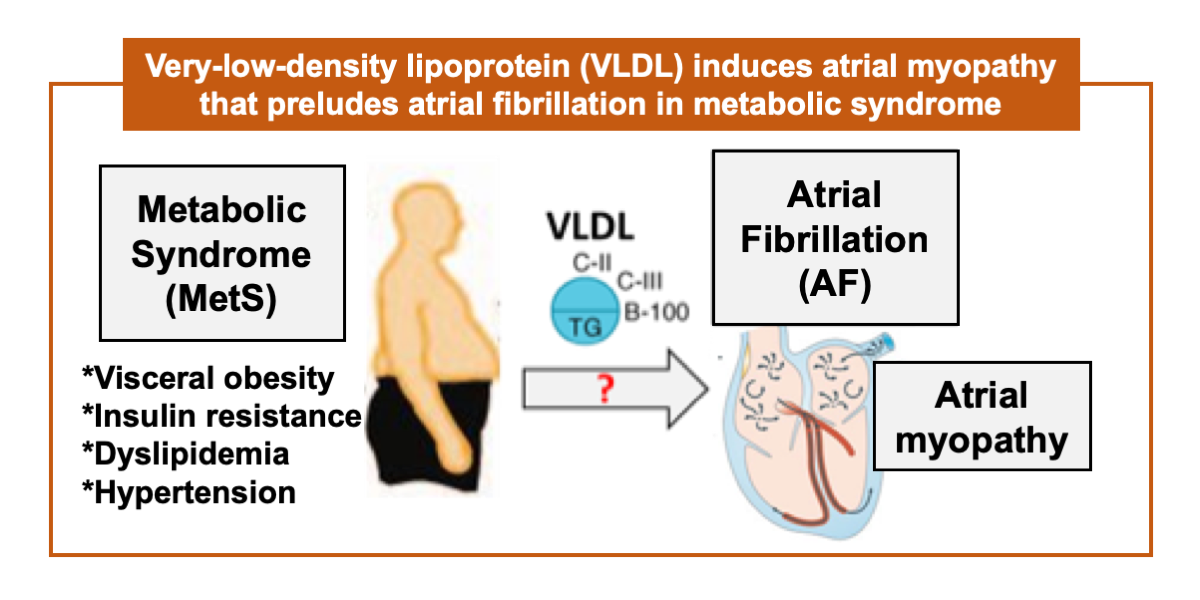
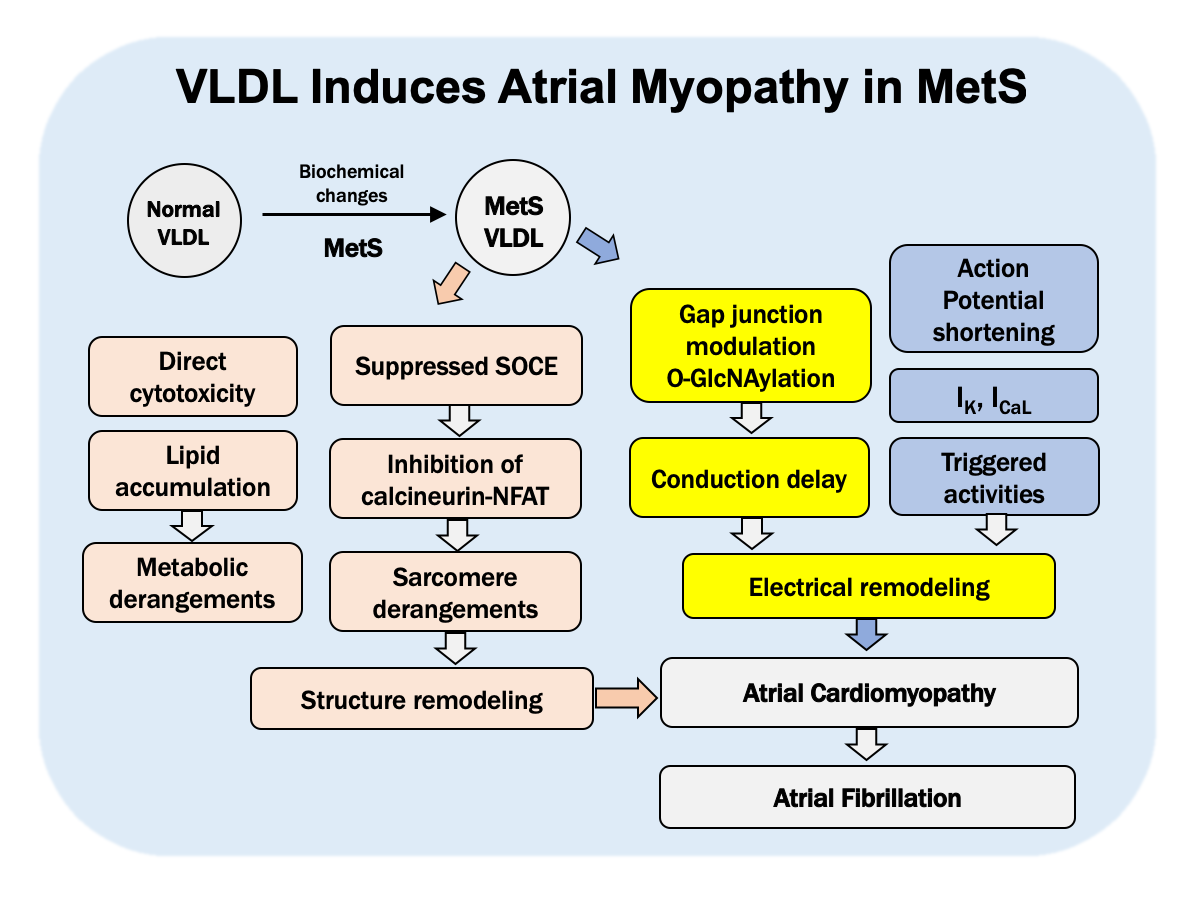
Atrial fibrillation can cause disabilities in the aged population, and it is preceded by atrial myopathy with one to several decades. Individuals with metabolic syndrome such as hypertension, insulin resistance, type 2 diabetes, elevated triglyceride, dyslipidemia, and central obesity commonly have coexisting atrial myopathy, and are at increased risk of incidence, and of the co-morbidities of atrial fibrillation. Among manifestations of metabolic syndrome, the lipid metabolism disorder frequently coincides with atrial fibrillation. Still, the relevance of pathological development of atrial fibrillation and hypercholesterolemia is ambiguous and controversial. Instead of cholesterol, very-low-density lipoprotein (VLDL) carries and circulates triglyceride in the bloodstream. We hypothesize that the triglyceride-enriched VLDL plays a pivotal role in the pathological development of atrial fibrillation under the metabolic syndrome. In our previous studies, we have evidenced that VLDL of individuals with metabolic syndrome has lipotoxicity to atrial tissue, and can cause atrial myopathy. VLDL regulates transcript-encoding of the gap-junction and gap-junction proteins, and the post-translational modification. Physiologically, VLDL delays intra-cardiac conduction. In addition, VLDL regulates modulation of STIM1, and then in turn suppresses store-operated calcium entry into the atrial myocytes, and down-regulates the calcineurin-NFAT pathway. Consequently, changes in myofilament proteins and sarcomere derangement appear in mouse atrial tissue. In a clinical study, our preliminary data showed that the postprandial electronegative VLDL is strongly concerned with atrial remodeling that arises from metabolic syndrome. An old proverb says “Illness enters by the mouth.” Overeating carries excessive and harmful VLDL that accelerates aging of the heart. Controlling VLDL metabolism or neutralizing the toxic effect of VLDL may be the way to lower the number of disabled people in the aged population.
Longevity is one of the many common things people wish for. The process of aging, however, accumulates odds for disabilities. Based on WHO global estimation, the disabled population is on the rise due to population aging and the rapid increase in chronic diseases. Among those diseases, atrial fibrillation (AF) is one of the major etiologies to cause disabilities.
AF is the most common persistent arrhythmia, and the incidence rate increases rapidly with the aging of the population. About 5% of the over 65-year-old population and 10% of the over 80-year-old population are diagnosed with AF, which presents as abnormally irregular atrial exhibition of electricity, and can lead to blood clots in the heart, causing stroke, arterial thromboembolism and heart failure. Asymptomatic atrial myopathy precedes AF by one to several decades. Patients of atrial myopathy are often underdiagnosed until AF arises with symptoms, including palpitation, breathlessness, or dizziness. Once AF occurs, commonly it gradually becomes persistent, then permanent as a gradual failure in control on anti-arrhythmic drugs or a relapse from anti-arrhythmic ablation therapy.
To reduce the number of disabled old people in our aging life, prevention of AF is an important task. The prevention must begin early in life. The atrial myopathy should be controlled to prevent the occurrence of AF. Individuals with metabolic syndrome (MetS) carry a higher risk for both the incidence and the comorbidities of AF. Therefore, the overarching goal of our research is to investigate the cellular and molecular mechanisms underlying pathological development of atrial myopathy in the MetS.
The MetS is multiplex risk factor linked to several clinical phenotypes, including hypertension, insulin resistance or type 2 diabetes mellitus, dyslipidemia, with elevated triglyceride and reduced high-density lipoprotein cholesterol level, and also central obesity. It is quite common to observe atrial enlargement in individuals with MetS. Since dyslipidemia is a key feature of MetS, clinical studies have investigated to see if there is any correlation between dyslipidemia and AF. However, the results are contradictory. Statin, the most commonly used cholesterol-lowering drug, can significantly reduce risk of AF. Nevertheless, when we survey clinical studies that pinpointed the correlation among lipid profiles and risk of AF, the association is inverse for LDL-C. In other words, low LDL-C is actually associated with higher risk of AF. We believe there is a pathological role of the lipid disorder acting in atrial myopathy. Moreover, we hypothesize that very-low-density lipoprotein (VLDL), carrying most insoluble triglyceride in our bloodstream, can cause atrial myopathy, especially in the MetS. This hypothesis has been corroborated by our experimental data and is ready to be articulated to a new pathogenesis theory in which VLDL promotes atrial myopathy and vulnerability to AF in MetS.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.