The Power of Triage (CADt) for patients with intracranial hemorrhage in the Emergency department

Author(s)
Jung Lung HsuBiography
Dr. Hsu is currently an associate professor in the Department of Neurology at Chang Gung Memorial Hospital in Linkou, Taiwan. He was also appointed to the Graduate Institute of Mind, Brain, and Consciousness, Taipei Medical University, and the Brain and Consciousness Research Center, TMU Shuang Ho Hospital, New Taipei City, Taiwan.
Academy/University/Organization
Taipei Medical UniversityEdited by
Jung Lung HsuSource
https://www.ncbi.nlm.nih.gov/pubmed/?term=Jung+Lung%2C+Hsu
https://www.ncbi.nlm.nih.gov/pubmed/29169407
https://www.ncbi.nlm.nih.gov/pubmed/28496183
https://www.ncbi.nlm.nih.gov/pubmed/22825428-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- August 21,2019
Currently, precision diagnosis of degenerative dementia based on the different misfolding proteins could be performed by state-of-the-art molecular imaging. Using amyloid and tau tracers in PET imaging could improve our knowledge of underlying neuropathologic changes in different types of dementia, and help us recognize the topographical distribution of misfolding proteins. These findings could move forward our understanding of dementia and its clinical associations.
Dementia is a clinical syndrome which is becoming markedly more common with the aging of Taiwan’s population. One of the common etiologies in dementia syndrome is Alzheimer’s disease (AD), which is pathologically defined by the amyloid-ß and tau deposition in the brain. Clinically, AD is a progressive neurodegenerative disorder with cognitive, behavioral and functional alterations. From past studies, more than 20 years will be needed for accumulation of amyloid-ß and tau proteins in the brain to reach the symptomatic stage of dementia. During this period, early detection of the above two important biomarkers will help clinicians with early recognition of or even early intervention in AD. In the past few years, our team at the Department of Neurology in Chang Gung Memorial Hospital, Linkou branch has established a cognitive measurement (Consortium to Establish a Registry for Alzheimer’s Disease Neuropsychological Assessment Battery) and a functional questionnaire (Everyday Cognition scale, ECog) with positron emission tomography (PET) scan in patients with AD. The results showed that ECog scores correlated with the cognitive measurement and neuroimaging biomarkers. Besides, using the fluid biomarkers from patients with AD, we found that the plasma clusterin level was correlated with dementia severity and right-side parietal lobe atrophy from brain scans. Meanwhile, the right-side parietal atrophy was correlated with agitation/aggression symptoms in patients with AD. From a longitudinal point of view, the plasma clusterin could serve as a biomarker for the severity of cognitive decline in these patients. Combined with fluid biomarkers, cognitive measurement could correctly estimate the AD severity and predict the future deterioration.
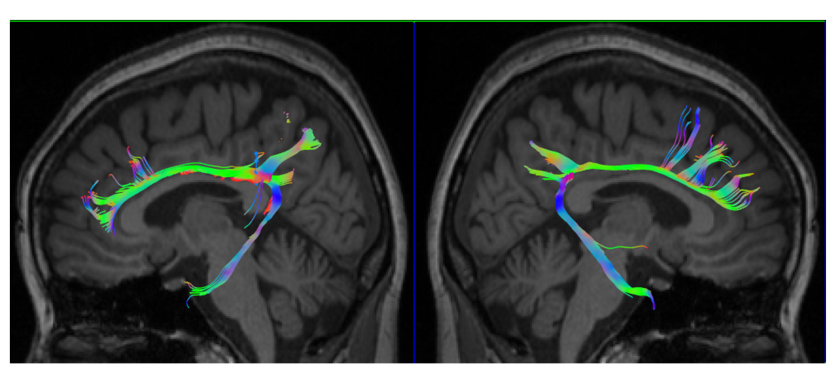
Besides, the study team from the Department of Neurology and Nuclear Medicine has been using the latest development of a molecular imaging tool (PET-MR) to explore the neurodegenerative diseases by multi-model imaging information. In terms of molecular imaging, using the dual-phase imaging method, we could estimate the perfusion state and trace-specific state in one scanning time. This method could provide both perfusion results and tracer-specific imaging for clinicians, improve the image coregistration method and decrease the exposure of radiation activity for patients. Using this method, we have developed a new algorithm to measure the dopaminergic system by the vesicular monoamine transporter type 2 (VMAT2) activities, which could accurately estimate the severity of Parkinson’s disease and differentiate the parkinsonism syndrome. Using the 18F-florbetapir (AV-45) amyloid PET imaging, we have explored the bio-distribution of this tracer, its correlation with cerebral metabolism and association with amnestic mild cognitive impairment and dementia. The study team also conducted the Taiwan Alzheimer’s disease neuroimaging initiative (T-ADNI) project and cooperated with four medical centers in Taiwan to build the research platform. Recently, the study team established cooperation with Tohoku University in Japan to use the THK-5351 tau tracer to explore familial AD. We have found increasing tau deposition in the cerebellar cortex in patients with familial AD (APP mutation). In terms of MR imaging, in cooperation with Utrecht University in the Netherlands, we found an association between the tau deposition and microstructural integrity measured by diffusion tensor imaging (DTI) in patients with vascular cognitive impairment. This finding suggests that tau deposition is related to the axonal changes in the peri-lesional area near the infarction zone. Finally, using amyloid PET and tau PET results, we correctly labeled 39 patients with young onset dementia by the ATN research framework proposed by the NIA-AA criteria. We firstly provided the ratios of different subtypes in young onset dementia based on the molecular imaging diagnosis.
Figure: A poster presentation at the 2019 Human Amyloid imaging conference on using the novel tau PET tracer to explore the image and clinical features in Alzheimer’s disease
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




