How Long Can Cancer Patients Live?
Author(s)
Chien-Hui Lo, I-Hsuan Lin, T. Tony Yang, Yen-Chun Huang, Barbara E. Tanos, Po-Chun Chou, Chih-Wei Chang, Yeou-Guang Tsay, Jung-Chi Liao, and Won-Jing WangBiography
Prof. Wang is current an associate professor in the Institute of Biochemistry and Molecular Biology at National Yang-Ming University in Taipei, Taiwan. She is also a jointed member at Taiwan International Graduate Program (TIGP) in Molecular medicine.
Academy/University/Organization
National Yang-Ming UniversitySource
https://www.ncbi.nlm.nih.gov/pubmed/31455668-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- February 20,2020
The primary cilium is an evolutionarily conserved organelle that plays important roles in development and tissue homeostasis. Tau-tubulin kinase-2 (TTBK2) is genetically linked to spinocerebellar ataxia type 11, and its kinase activity is necessary for ciliogenesis. However, little is known about the TTBK2 physiological substrates associated with ciliogenesis. In our lab, we use direct stochastic optical reconstruction microscopy (dSTORM) superresolution microscopy to analyze TTBK2 distribution at the centrioles during ciliogenesis, and we have discovered that ciliogenesis changes the TTBK2 signal from the periphery toward the root of the centriole distal appendages, where TTBK2 colocalizes with CEP83. Our biochemical analyses therefore indicate that CEP83 is a bona fide TTBK2 substrate. Using genetically edited cell lines that lack TTBK2-dependent CEP83 phosphorylation, we further demonstrate that CEP83 phosphorylation mediated by TTBK2 is essential for the early ciliogenesis steps including ciliary vesicle docking and subsequent CP110 removal. In brief, our results first characterize a centriole-related substrate for TTBK2 and also provide a new narrative regarding ciliogenesis supported by the interplay between TTBK2 and CEP83 at the centriole.
The primary cilium is a membrane-bound structure with a microtubule-based core and is present in most cells in our body. It serves as a sensory hub for the transduction of extracellular signals to regulate gene expression, thus playing important regulatory roles during proliferation and tissue homeostasis. It is known that two factors are essential for cilia formation. First, it requires the presence of a pinwheel-like structure protruding from the distal end of the centriole, named centriole distal appendages. To date, several proteins at the distal appendages have been identified, including CEP164, SCLT1, CCDC41/CEP83, CCDC123/CEP89, FBF1, and LRRC45. Their recruitment to the centriole is known to be hierarchical. CEP83 is first recruited to the centrioles and is required for the recruitment of SCLT1 and CEP89. SCLT1 is necessary for the subsequent recruitment of CEP164 and LRRC45. Using direct stochastic optical reconstruction microscopy (dSTORM), CEP83, CEP89, SCLT1 and CEP164 have been shown to form the backbone of the centriole distal appendages. CEP83 is located at the root and CEP164 is at the periphery of the centriole distal appendages. Another essential factor required for ciliogenesis is the Tau-tubulin kinase-2 (TTBK2). TTBK2 is a serine/threonine protein kinase originally identified as a microtubule-associated protein phosphorylating tau and tubulin. Although it has been demonstrated that TTBK2 activity is essential for cilia initiation, TTBK2 substrates associated with its role in ciliogenesis have not yet been identified.
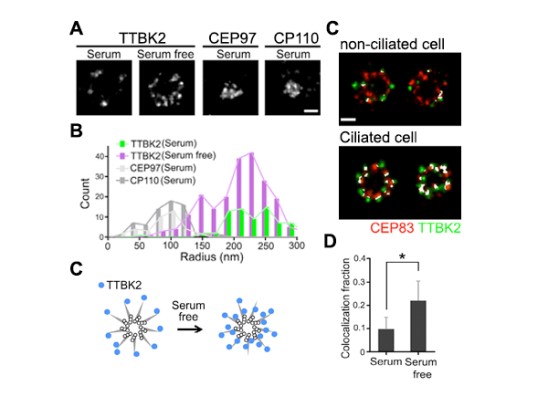
Figure 1: TTBK2 is enriched toward the axoneme, and colocalizes with CEP83 during ciliogenesis.
In our lab, we used dSTORM superresolution microscopy to examine TTBK2 signals at the centriole during ciliogenesis. The axial-view of the dSTORM images revealed that the TTBK2 signals were sporadically scattered at the distal portion of the mother centriole in unciliated cells. However, the TTBK2 signal redistributed and formed a smaller ring around the mother centriole in ciliated cells (Figure 1A-1C). The changes in the TTBK2 signal at the centriole in responding to ciliogenesis suggested that TTBK2 redistribution at the onset of ciliogenesis might be required for TTBK2 to reach its substrate(s) in promoting cilia initiation. We then analyzed the TTBK2 signal with the proteins at the centriole to identify the TTBK2 substrate. Our dSTORM images showed that cilia formation resulted in a larger population of TTBK2 colocalized with CEP83 (Figure 1D & 1E). It also suggested that CEP83 was a potential substrate of TTBK2.
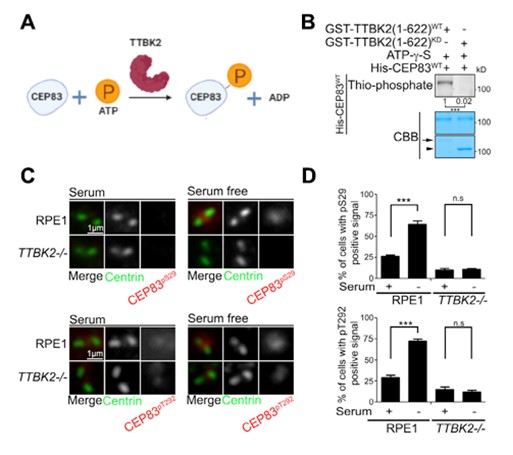
Figure 2: CEP83 is phosphorylated by TTBK2 during ciliogenesis.
To explore the kinase-substrate relationship between TTBK2 and CEP83, we set up TTBK2 in vitro kinase assays using recombinant TTBK2 and CEP83 purified from bacteria. TTBK2 was able to phosphorylate CEP83 in the in vitro kinase assay, suggesting that CEP83 was a direct substrate of TTBK2 (Figures 2A & 2B). We next attempted to identify the sites on CEP83 that were phosphorylated by TTBK2 using tandem mass spectrometry analysis. Our results showed that TTBK2 phosphorylated CEP83 at Ser29, Thr292, Thr527, and Ser698. A phospho-inactive mutant of CEP83 (CEP834A) was then generated to examine the effect of TTBK2 on CEP83 by mutating CEP83 residues S29, T292, T527 and S698 to alanine. The in vitro kinase assay showed that TTBK2 was unable to phosphorylate CEP834A, further supporting that TTBK2 phosphorylated CEP83 at Ser29, Thr292, Thr527 and Ser698.
Two phospho-CEP83 antibodies (anti-phospho-CEP83S29 and anti-phospho-CEP83T292) were then generated to examine endogenous CEP83 phosphorylation during ciliogenesis. By performing immunostaining using two phospho-CEP83 antibodies, our results showed that the phospho-CEP83 signals were much higher in ciliated cells than in non-ciliated cells (Figure 2C & 2D). We also generated TTBK2 knockout cells to examine CEP83 phosphorylation during ciliogenesis. In the TTBK2 knockout cells, CEP83 phosphorylation was not induced during ciliogenesis (Figure 2C & 2D). Together, our results indicated that CEP83 phosphorylation was induced during ciliogenesis and was mediated by TTBK2.
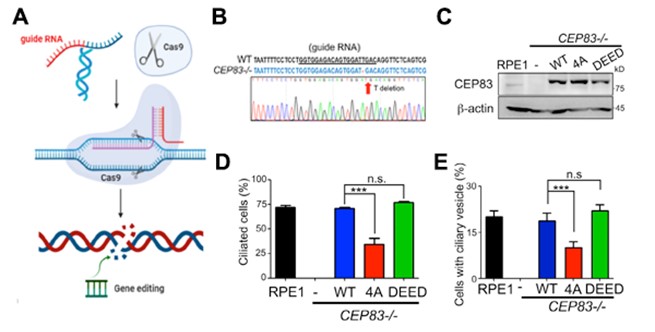
Figure 3: TTBK2-dependent CEP83 phosphorylation promotes cilia formation at the initial stage.
To further study the role of CEP83 in ciliogenesis, we generated CEP83 knockout cells (Figure 3A & 3B). Using three-dimensional structured illumination microscopy (3D-SIM), we found that depletion of CEP83 completely blocked the formation of ciliary vesicles, indicating the importance of CEP83 in the control of ciliary vesicle docking during ciliogenesis (Figure 3E). To examine the effect of CEP83 phosphorylation in ciliogenesis, CEP83WT, CEP83 phospho-inactive (CEP834A), or CEP83 phospho-mimic (CEP83DEED) was stably expressed in CEP83 knockout cells. CEP83WT, CEP834A, and CEP83DEED expressing cell lines were established using single cell cloning. Both CEP834A and CEP83DEED were expressed equally and localized properly to mother centrioles (Figure 3C). We then checked the effects of CEP83 phosphorylation in ciliogenesis. By comparison with CEP83WT expressing cells, the percentage of cells which formed cilia dramatically reduced in the CEP834A expressing cells, but not in the CEP83DEED expressing cells (Figure 3D). To understand how CEP83 phosphorylation contributed to ciliogenesis, we analyzed the key steps during ciliogenesis. Our results showed that CEP83 phosphorylation affected the formation of ciliary vesicles during ciliogenesis and the removal of CEP110 from the mother centriole (Figure 3E). Thus, our results suggest a model where TTBK2-dependent CEP83 phosphorylation is important for ciliogenesis by regulating membrane vesicle docking that promotes CP110 removal from the mother centrioles (Figure 4).
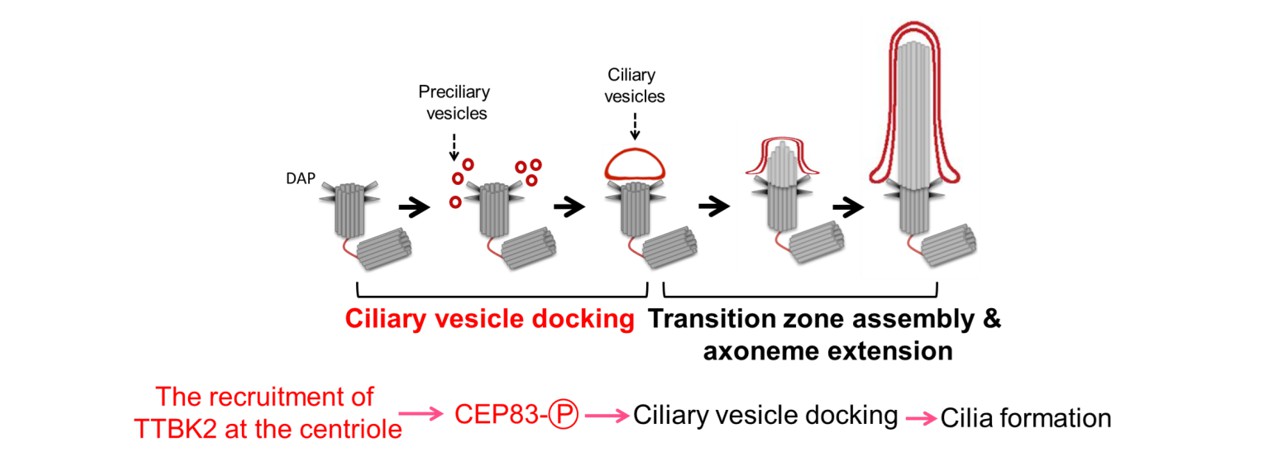
Figure 4: The model shows that phosphorylation of CEP83 by TTBK2 is essential for cilia initiation.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




