Supply Chain Security Threats Hunting
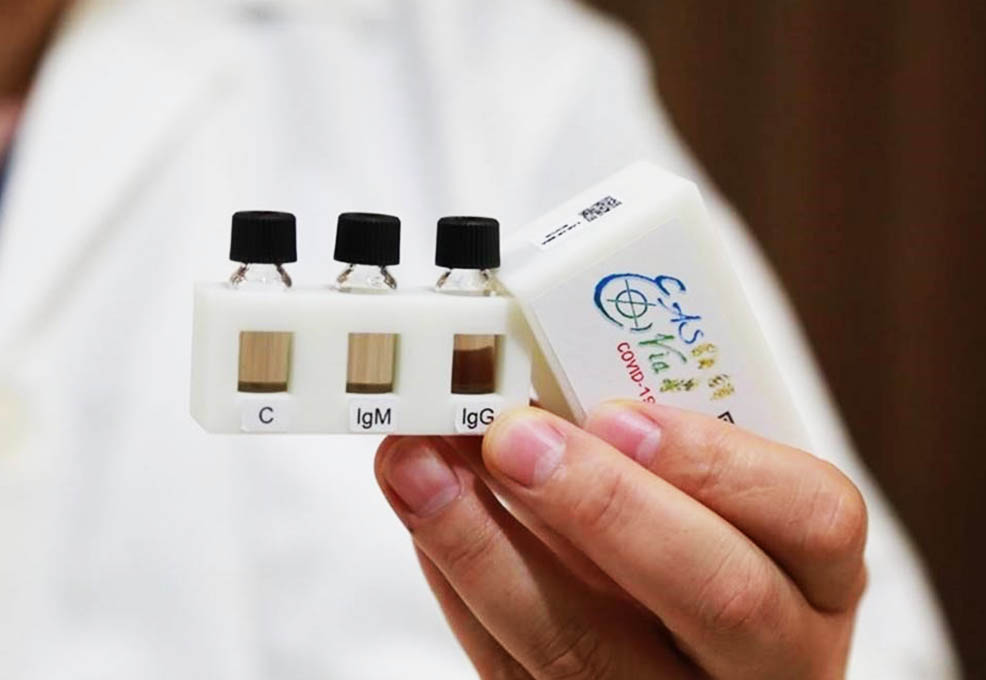
The ultrasensitive and rapid diagnostic testing kit.
Author(s)
Hung-Wei YangBiography
Dr. Hung-Wei Yang is currently an associate professor at National Sun Yat-sen University, Taiwan, which he joined in 2014, and was recently selected as a Distinguished Young Scholar. He has received the Excellent Young Investigator Research Grant two times from Taiwan’s Ministry of Science and Technology, the Outstanding Paper Award three times from the Association of Chemical Sensors in Taiwan, and the Excellent Academic Research Award two times from National Sun Yat-sen University. His general research interests focus on developing nanobiomaterials, virus-like particle delivery systems, microneedle patches for drug/vaccine delivery, and smart biosensing systems for public health.
Academy/University/Organization
National Sun Yat-sen University-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- ENGINEERING & TECHNOLOGIES
- Text & Image
- January 15,2021
As the COVID-19 pandemic is spreading around the world, fast and accurate detection technology is indeed an important requirement. However, the current quantitative real-time reverse transcription polymerase chain reaction assay (qRT-PCR) is time-consuming, high-cost and requires medical specialists to collect samples and perform sample testing. This will decrease the testing efficiency in the face of suspected infections with no obvious symptoms. In light of this, our team aims to develop an ultrasensitive and rapid diagnostic technology for dual-antibody testing to solve the difficulties of epidemic prevention caused by emergency infectious diseases. We have now completed the development of the prototype and verified it with clinical specimens. The results confirmed that only a drop of fingertip blood can quickly detect the IgM/IgG antibodies in the blood in the early stage of COVID-19 infection within 15 minutes. Compared with the lateral flow immunoassay (LFIA), its detection sensitivity can be greatly improved by 6,000 times from 65 ng/mL to 0.01 ng/mL. The ultrasensitive and rapid diagnostic test can be further applied to detect communicable diseases caused by viruses such as Zika, dengue, and Ebola. The test can come in handy during virus spreading seasons or in the case of an epidemic outbreak, greatly reducing the engagement of medical personnel and preventing the spread of the epidemic.

Dr. Yang’s research team and his collaborators from Kaohsiung Medical University.
The spread of COVID-19 around the world had caused more than 20 million infections and more than 700,000 deaths by the end of August 2020. During the outbreak of infectious disease, tests to detect infected patients are urgently needed. The current quantitative real-time reverse transcription polymerase chain reaction assay (qRT-PCR) is time-consuming, high-cost and requires medical specialists to collect samples and perform sample testing, making it impossible to screen and isolate suspected infected persons in time considering the rapid spread of the epidemic. To interrupt the virus transmission, a rapid, sensitive and simple diagnostic test is urgently required.
The most common rapid test for IgM or IgG antibodies with lateral flow immunoassay (LFIA) strips could provide infection information for disease treatment and prevention without additional facilities or instrumentation with a turnaround time of 10-20 min, making it of particular value for infectious disease outbreak control in regions with insufficient medical resources. Because of the poor detection sensitivity of the LFIA strips (65 ng/mL), they cannot detect the relatively low concentration of IgM antibody in the blood of the initial infected persons, so false negative results often occur. In light of this, the research team of associate professor Hung-Wei Yang from the Institute of Medical Science and Technology of National Sun Yat-sen University developed an ultrasensitive and rapid testing kit based on highly catalytic nanomaterial and biomolecular patterning techniques that differ from LFIA strips. No medical personnel are needed in the process: only a drop of fingertip blood is enough to detect IgM and IgG antibodies in a patient in an early stage of COVID-19 infection showing no obvious symptoms in just 15 minutes. Compared with the LFIA strips, the detection sensitivity can be greatly improved by 6,000 times from 65 ng/mL to 0.01 ng/mL.
The ultrasensitive and rapid testing kit has undergone a blind test on clinical blood samples from healthy people, people who have been infected by other viruses, and people who have been tested as COVID-19 positive by an qRT-PCR. The testing results all showed negative (no yellow color observed) for the healthy people and the people who had been infected by the Hepatitis B, Hepatitis C, or influenza viruses (Figure 1). Conversely, the testing results showed positive for the patients who had been diagnosed with COVID-19 by qRT-PCR. Interestingly, we also conducted a blind test using the testing kit on a patient who returned to Taiwan from abroad and showed no obvious symptoms but was tested positive by qRT-PCR. We can accurately detect the trace IgM antibodies but no IgG antibodies within 15 minutes in the blood of the first day of hospitalization of the patient, indicating that the testing kit can be used to find suspected infections with no obvious symptoms in an early stage of infection (the test allows the detection of IgM antibodies even with 10,000 times smaller concentration). The IgG antibodies can be significantly detected in the seventh day of hospitalization, indicating that the testing kit can be used to assess the history of infection and track the distribution of infected areas. Dr. Yang pointed out that the developed ultrasensitive and rapid testing kit can be further applied to detect other infectious diseases caused by viruses such as Zika, dengue, and Ebola, greatly reducing the engagement of medical personnel and preventing the spread of the epidemic. The team has also filed an application with the Taiwan Food and Drug Administration (TFDA) and U.S. FDA for emergency use authorization (EUA) of the testing kit, based on the results of the clinical trials.
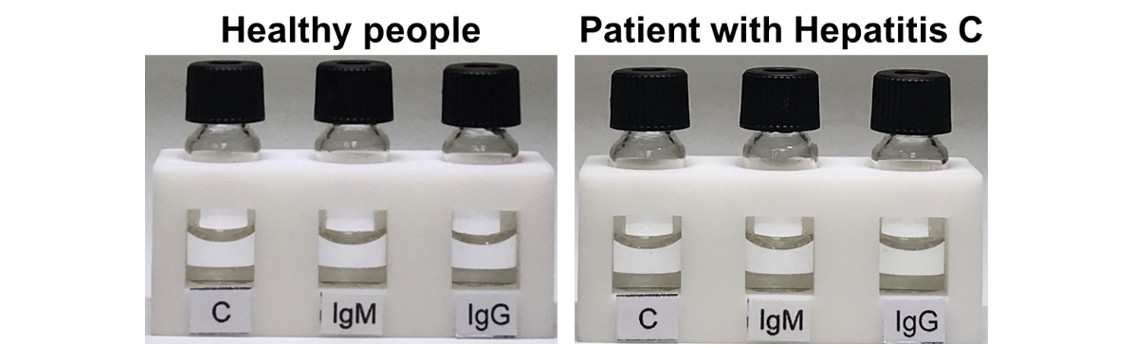
Figure 1. The testing of blood samples from healthy people and a patient infected with the Hepatitis C virus; the testing results were both negative.
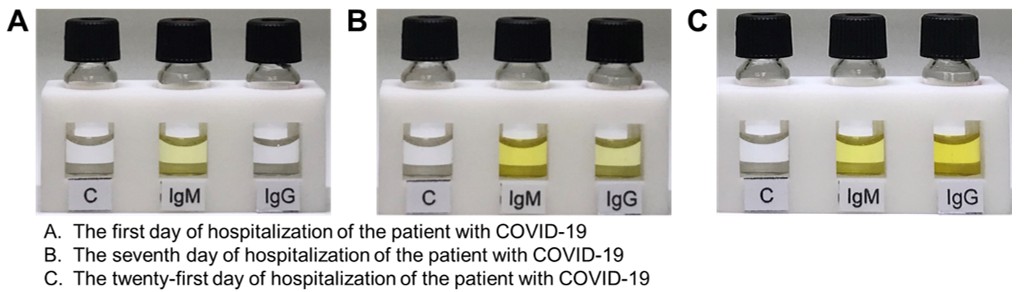
Figure 2. The testing of blood samples from a patient who returned to Taiwan from abroad and showed no obvious symptoms but tested positive by qRT-PCR. The IgM antibodies can be detected in the blood on the first day of hospitalization, and the IgG antibodies can be further detected in the blood on the seventh day and twenty-first day of hospitalization.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




