Sensors-The Senses of Smart Machinery

Author(s)
Nae-Lih WuBiography
Dr. Nae-Lih Wu is a Distinguished Professor in the Chemical Engineering Department at National Taiwan University (NTU). His research interests include materials for electrochemical energy-storage devices, including supercapacitors and rechargeable batteries, advanced in-situ/in-operando synchrotron analytic methodologies, and nano-materials synthesis and applications. He is currently an associate editor of Journal of the Electrochemical Society.
Academy/University/Organization
National Taiwan University-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- ENGINEERING & TECHNOLOGIES
- Text & Image
- March 20,2020
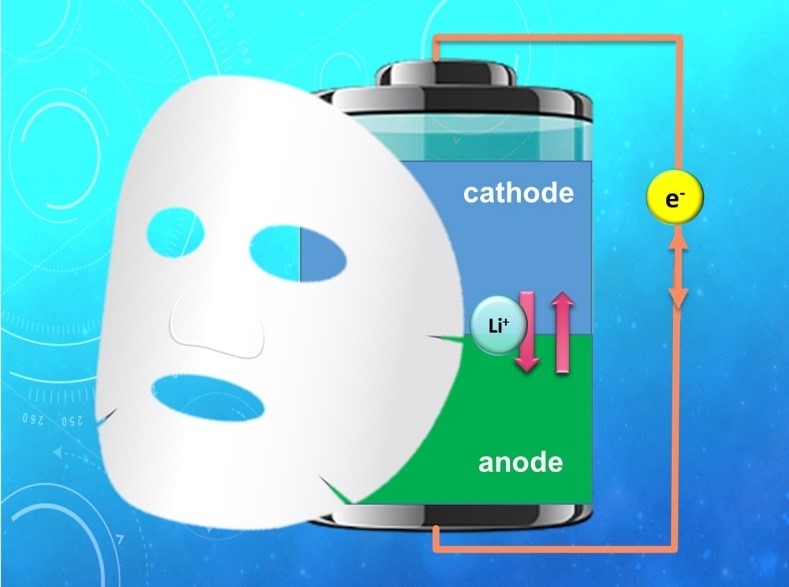
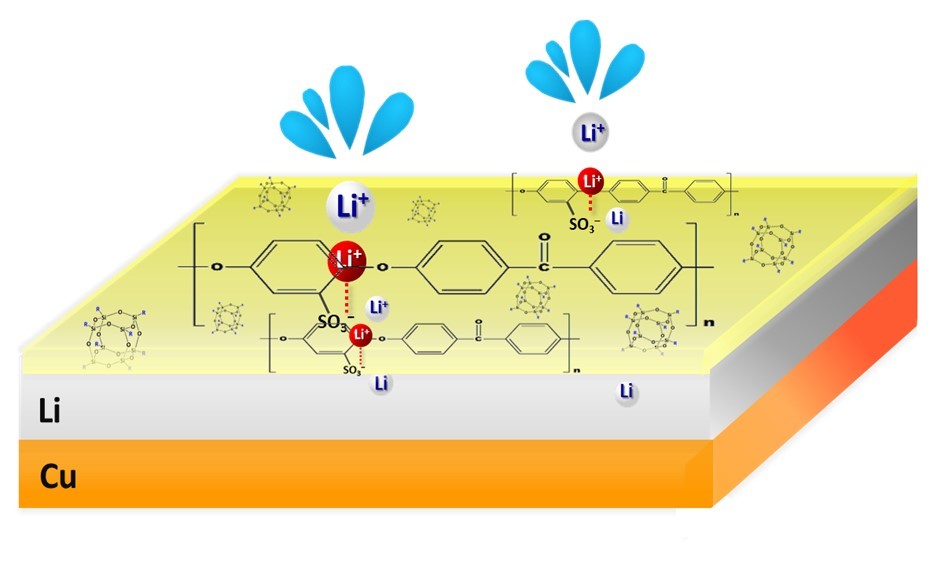
A low-cost and high-capacity battery system for energy storage is crucial to the development of the green energy industry. Lithium-ion batteries are foreseen to be the major battery technology for the next decades. Although the theoretical charge-storage capacity of a battery is determined by the bulk properties of its electrode active materials, its various practical performance indices, such as practical capacity, charge-discharge rate capability, and cycle stability and safety, heavily depend on the properties of the electrolyte-active material interface. To tailor the interface with beneficial properties, we have been promoting the concept of applying a pre-coating of polymeric soft materials with designed functional groups onto the surfaces of the active materials. In a way, the coatings behave like cosmetic facial-masks to “condition” the properties of the surfaces of the electrode active materials. As an example, we have recently successfully fabricated an ultrathin ( ≦ 100 nm) “facial-mask” on a high-capacity Li anode to effectively suppress dendrite formation and enable extended charge-discharge life with high efficiency. The coating is an organic-inorganic composite ionomer membrane that expels anions in the electrolyte and allows only Li ions to pass through. The Li-ion-selective property of the “facial-mask” helps to evenly distribute Li ions over the entire electrode surface so that formation of dendrite can be avoided. Meanwhile, the organic polymer matrix possesses the high mechanical strength and dimensional rigidity needed for long-life operation. This invention paves the way toward the realization of Li-metal anode technology for advanced high-capacity Li-ion batteries.
In view of the impact of global warming on the environment, the green energy industry has been listed as a key industry for future development around the world. For renewable energy sources, such as solar energy and wind power, the most important part in the development of the power generation systems is the availability of a low-cost and high-capacity battery system for energy storage. Meanwhile, the key to the success of the electric vehicle industry also relies on the development of battery technology. Lithium-ion batteries are foreseen to be the major battery technology for the next decades. Effectively enhancing the performance of battery materials of lithium-ion batteries can certainly boost the future development of green energy technology.
Li-ion batteries consist of positive and negative electrodes that can accommodate Li ions in different energy states (Figure 1). Li ions shuttle between the two electrodes during charge and discharge to store or release electric currents, respectively. Although the theoretical charge-storage capacity of a battery is determined by the bulk properties of its electrode active materials, the various practical performance indices, such as practical capacity, charge-discharge rate capability, cycle stability and safety of the battery, heavily depend on the conditions of the interfaces between the electrolyte and the active materials. In the traditional method for modifying the interface properties for better battery performance, chemical additives are introduced into the electrolyte, where they undergo redox reactions and form coatings on the active material surfaces upon charge/discharge of a battery. However, the composition and structure of such an interfacial coating is determined by very complex and very often unpredictable relations among the additives, parent electrolyte, and charge-discharge protocols. The additives may not be sustainable but are consumed during the course of battery operation. In 2015 we proposed a new approach where polymeric soft coatings with tailored functional groups are applied to the surfaces of the active materials before they are subjected to electrode manufacturing. The compositions and structures of the coatings can be pre-designed with oriented function(s) for each of its components. In a way, the coatings may behave like cosmetic facial-masks to “condition” the properties of the active-material surfaces. The pre-design concept enables the selection of a wide variety of chemical compositions containing different combinations of organic and inorganic materials.
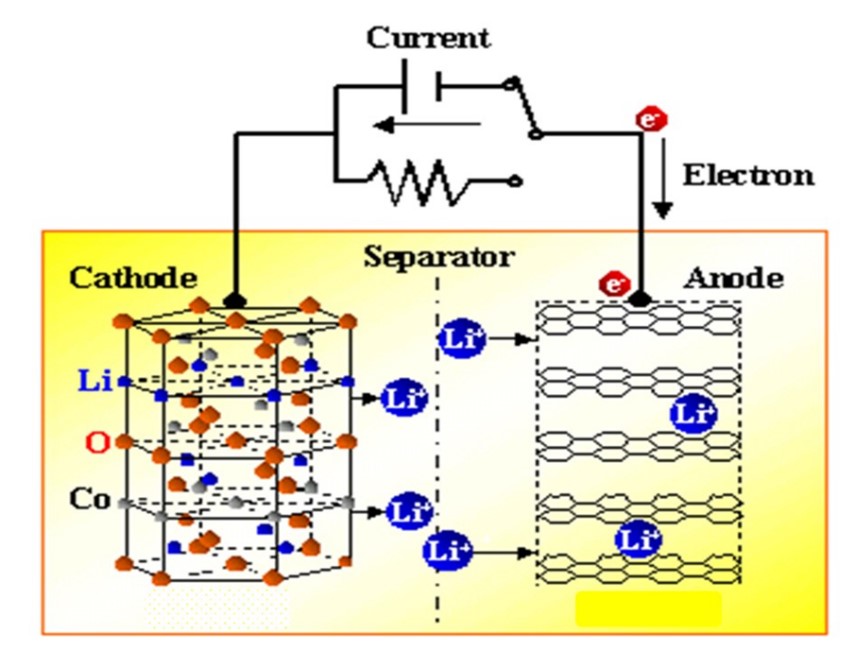
Figure 1. Schematic diagram showing the working principle of a Lithium-ion battery.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.