Save Papaya: Biotechnology Solutions for Viral Infection Control
Author(s)
Guan-Da SyuBiography
Dr. Syu is currently an assistant professor in the Department of Biotechnology and Bioindustry Sciences at National Cheng Kung University in Tainan, Taiwan. His research focuses on protein microarray technology and its application to the profiling of immune responses, specificity of the biologicals, protein-molecule interactions, and drug screening.
Academy/University/Organization
National Cheng Kung UniversitySource
https://money.udn.com/money/story/5635/4644729-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- LIFE SCIENCES
- Text & Image
- January 15,2021
Coronavirus is an enveloped RNA virus which causes several respiratory diseases in humans, including Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and most recently Coronavirus disease 2019 (COVID-19). Due to the recent outbreak of COVID-19, it is important to identify the COVID-19 patients in order to limit the viral transmission. The current detection method is based on the real-time RT PCR on the respiratory specimens which could be limited by the local viral load. Analysis of the humoral immune responses against coronavirus antigens would be one of the complementary methods to identify the COVID-19 patients because humoral immune responses are systematic and last for a long period of time. In order to gain full understanding of the humoral immunity in COVID-19 patients, we developed the coronavirus protein microarray that includes proteins from SARS, MERS, and COVID-19. We printed each protein in triplicate and formed 14 identical blocks per array. We showed the high sensitivity of this protein array with detection limits lower than 50 pg. By profiling the serum IgG and IgM from 33 COVID-19 patients and 35 controls, the array demonstrated 97% sensitivity and 97% specificity, exceeding the FDA requirement. Moreover, we discovered a set of biomarkers for COVID-19 diagnosis which can be translated into a rapid test. Overall, the coronavirus protein array is multiplexed, sensitive, specific, and flexible, and can be useful in diagnosis, disease assessment, and biological development.
At present, nasopharyngeal specimens are often collected for nucleic acid detection if patients are suspected of SARS-CoV-2 infection. This nasopharyngeal swab not only causes discomfort to the subjects, but also increases the risk of infection to the medical practitioner. According to the Shenzhen Hospital Research in China, only 59% of people were diagnosed by the first nucleic acid test. Therefore, it is often necessary to take multiple tests to confirm infection. Other than the nucleic acid test, serological tests are one of the most preferred methods because they detect the immunological responses against SARS-CoV-2 which can be detected in the blood from 7 days of infection to several months after recovery. However, most of the serological tests detect based on one viral protein, and result in insufficient accuracy due to the variety of the immune responses.
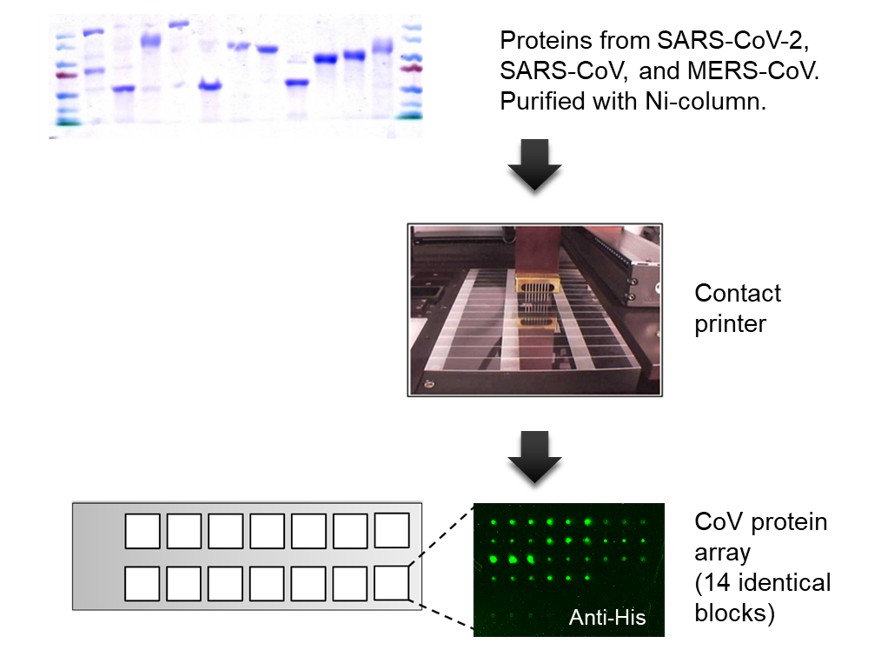
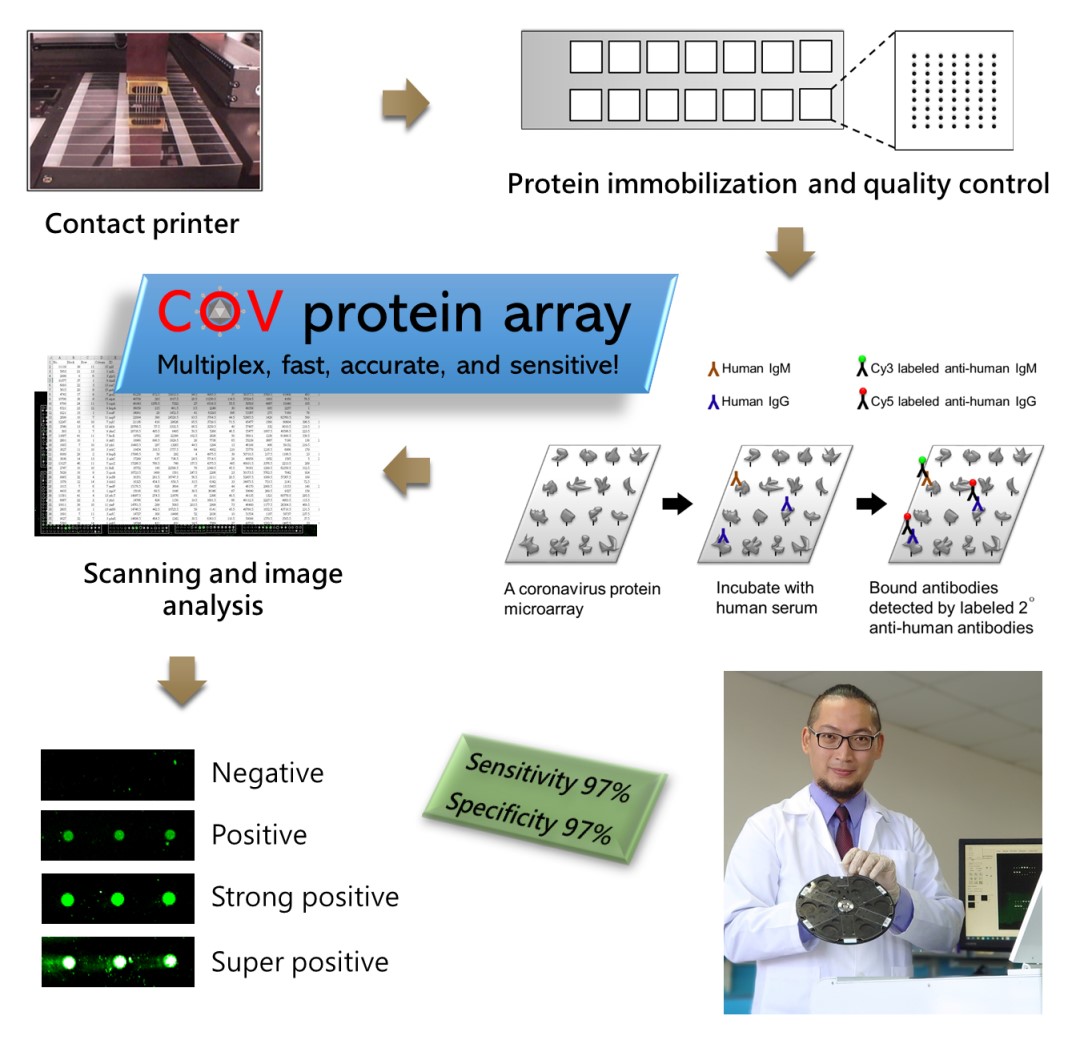
Figure 1. Fabrication of a coronavirus protein microarray. Proteins from SARS-CoV-2, SARS-CoV, and MERS-CoV were expressed in eukaryotic cells, purified via 6xHis-tag, and printed in triplicate on an aldehyde slide with 14 identical blocks. The quality of the coronavirus protein microarray was monitored by anti-6xHis fluorescence staining. All the purified protein showed positive anti-6xHis signals.
A protein microarray is a high-throughput screening platform which is very suitable for analyzing humoral immune responses. During the outbreak of the new coronavirus in January, Assistant Professor Guan-Da Syu, from the Institute of Biotechnology and Industrial Sciences in National Cheng Kung University, began to design the coronavirus protein microarray. The prototype was completed in February and tested in March (Figure 1). Through a series of production and quality controls, the coronavirus protein array with 100% protein expression and 25-50 pg antibody detection limit was built (Figure 2). The detection limit is 1,000-fold more sensitive than rapid tests or ELISA methods, which can greatly increase the information harness from blood, and reduce the sampling amount. The team further used clinical specimens to examine the diagnosis ability, by measuring IgG and IgM in the blood of 33 COVID-19 patients and 35 healthy subjects, obtaining 97% sensitivity and 97% specificity (Figure 3). Each coronavirus protein microarray can detect 14 blood samples at a time, with an average of 12 minutes per sample. Since there are many viral proteins in each array, they further narrow down to a set of biomarkers that can be used to produce a new generation of accurate rapid tests.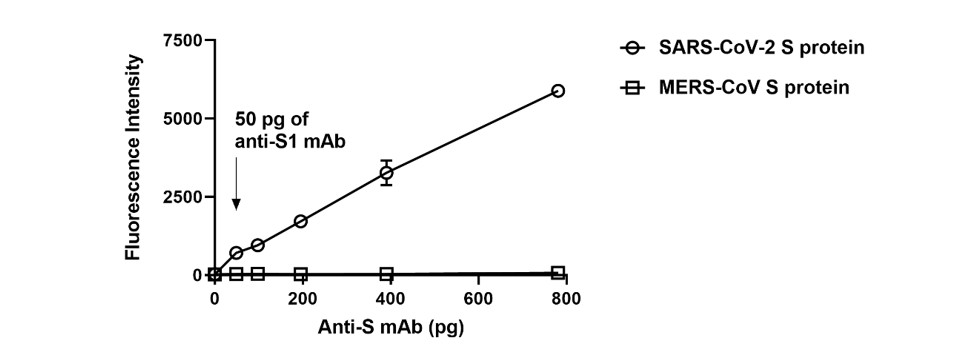
Figure 2. Detection limits of the coronavirus protein microarray. The detection limit of the coronavirus protein microarray was quantified by anti-S1 antibody. There is a wide linear range of S protein in SARS-CoV-2 but no response in MERS-CoV. The detection limit is <50 picograms of anti-S1 antibody.
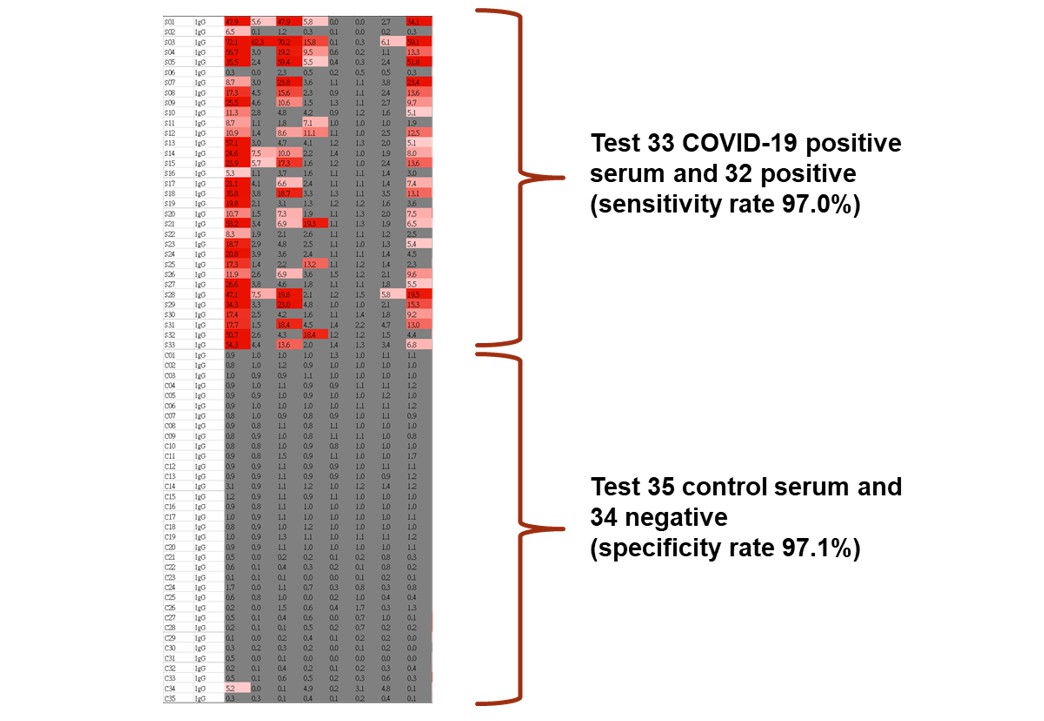
Figure 3. Serological tests using the coronavirus protein microarray. We profiled serum IgG and IgM responses against SARS-CoV-2 N and S proteins in 33 COVID-19 patients and 35 control subjects. The serology differences between the COVID-19 and control subjects were quite significant.
The protein chip technology was originally learned by Assistant Professor Guan-Da Syu from Heng Zhu’s laboratory, Johns Hopkins, the United States. In order to contribute to his home town, he returned to Taiwan six months ago and encountered this COVID-19 pandemic. With his expertise, he rapidly built and fabricated a coronavirus protein array, and recently his technology has been licensed to the world's largest human protein chip company, CDI Laboratories in the United States, and has been sold in Europe and the United States. He is grateful for the support from the NCKU incubation center and the dream plan for the budget support, Professor Chien-Sheng Chen for the equipment support, Dr. Yi-Ling Lin, Dr. Tzong-Shiann Ho, and Yi-Yiu Chou for the clinical assistance and guidance, and students Pinxian Du and Batuhan Keskin for their efforts. The coronavirus protein microarray has entered the stage of mass production and technology authorization. The automatic diagnostic systems for protein microarray and new-generation fast screening reagents are the two directions in the future (Figure 4). He believes the coronavirus protein microarray has great potential in both clinical and pharmaceutical applications.
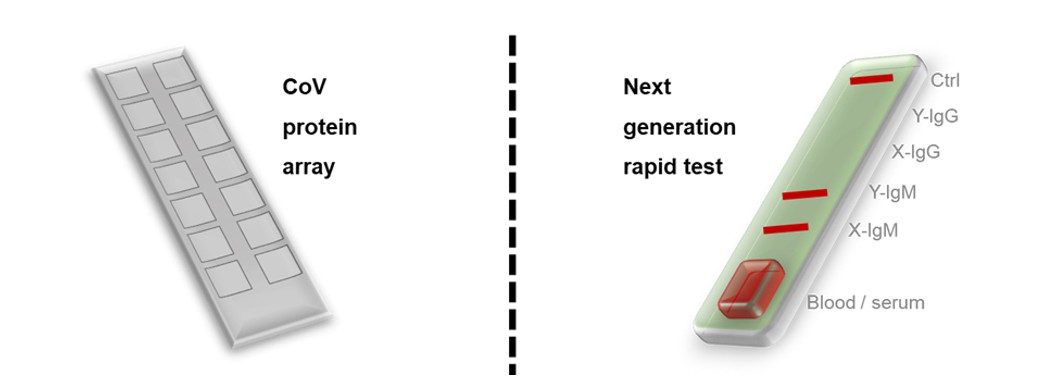
Figure 4. Products for the COVID-19 blood tests. The two related products were developed, i.e., the coronavirus protein microarray and the next generation rapid test based on the biomarker that found the COVID-19 serum profiling.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.