Magic Writing for Wearable Electronics
Author(s)
Yu-Jui FanBiography
Dr. Yu-Jui Fan is currently an Assistant Professor in the School of Biomedical Engineering, Taipei Medical University. His research interests include understanding ion concentration polarization mechanisms in microfluidic devices, the development of portable biosensing systems, and the integration of preconcentrating devices with biosensors for highly efficient biomedical and environmental detection.
Academy/University/Organization
Taipei Medical UniversitySource
https://www.sciencedirect.com/science/article/abs/pii/S221128551931122X?via%3Dihub-
TAGS
-
Share this article
You are free to share this article under the Attribution 4.0 International license
- ENGINEERING & TECHNOLOGIES
- Text & Image
- January 21,2020
Preconcentration of biomolecules for detection on microfluidic platforms based on electrical kinetic trapping (EKT) through ion concentration polarization (ICP) has been well developed in the past decade. Biomolecules can be entrapped due to the equilibrium of forces between electro-osmosis and ICP when applying a voltage to the system. However, the voltage required to trigger ICP phenomena in microfluidics is normally 30~80 V DC, which is a barrier to ICP-based nanofluidic preconcentrators becoming portable point-of-care devices. In this study, we developed a triboelectric nanogenerator (TENG)-driven nanofluidic preconcentrating device that is able to trigger ICP and subsequently cause the EKT of the biomolecules without using a conventional electrical power source. The EKT and electrical characteristics of the TENG-based nanofluidic preconcentrator were studied. Furthermore, the TENG-based nanofluidic preconcentrator was integrated with a smartphone-enabled bead immunoassay to become a portable and highly sensitive biosensor. The preconcentration and trapping processes were developed, and the bead-immunoassay on a smartphone with/without preconcentration was demonstrated to determine the preconcentration factors.
As Melinda Gates, the co-founder of the Bill & Melinda Gates foundation, said, “Whatever the conditions of people’s lives, wherever they live, however they live, we all share the same dreams.” In fact, the Bill & Melinda Gates Foundation works with organizations around the world that are using innovative methods to improve healthcare. How to rapidly screen acute diseases in developing countries where there is insufficient electric power is a critical issue. The enzyme-linked immunosorbent assay (ELISA) is the conventional technique to evaluate either the presence of antigens or antibodies in a sample. It is a useful tool for determining serum antibody concentrations through multiple steps and colorimetric analysis. An electric reader is necessary for analyzing ELISA results precisely. Further, because ELISA requires multiple steps, for targeting samples with low concentration (ng/ml level), the process time will be very long. To overcome the barrier, several label-free high sensitive biosensing devices have been developed, such as surface plasmon resonance (SPR), quartz crystal microbalance (QCM), and the cantilever beam. Therefore, most of the sensing devices still need a data acquisition system for highly sensitive detection and analysis.
We developed a bead-based immunoassay, which uses immunobeads to increase the association rate. The individual beads modified with antibodies are suspended in a sample solution. When the antibodies conjugate with the antigens, the immumobeads’ Brownian motion will decrease. Based on this phenomenon, by monitoring the decrease in the immunobeads’ Brownian motion, the concentration of antigens in the sample can be obtained. We also developed a smartphone-based microscopy that is able to monitor immunobeads’ Brownian diffusion. By measuring immunobeads’ diffusion coefficient in different concentrations of the antigen sample, in the equilibrium state, when the concentration of antigens was higher, a smaller diffusion coefficient was obtained. Additionally, we observed that diffusion coefficients of the immunobeads at antigen concentrations of 8.4 and 10.5 μg/ml were similar. We estimated that the number of antigens conjugating with the immunobeads had become saturated. Sensing results of antigen samples with concentrations in a linear range of 1.05~8.4 g/ml were found.
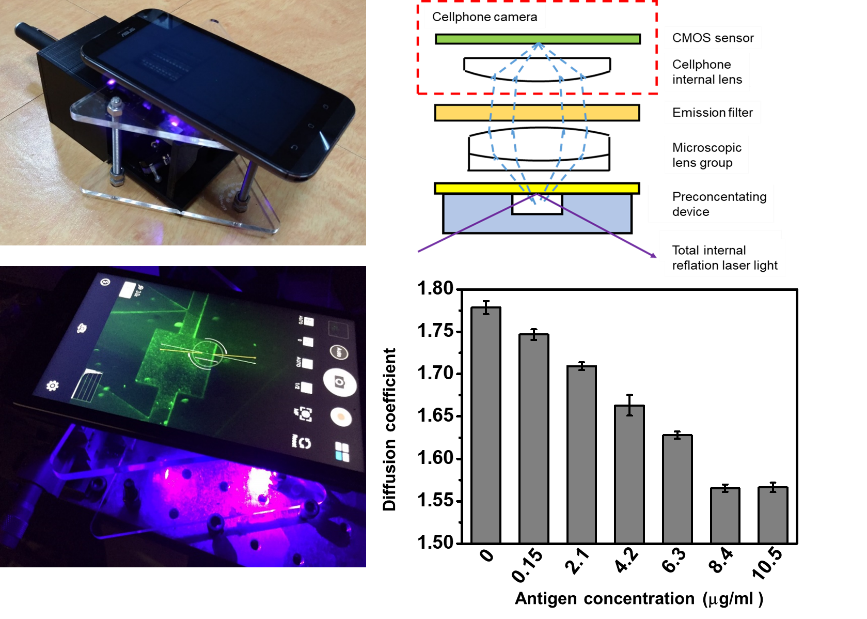
Figure 1. A smartphone-based microscopic system for observing immunobeads’ diffusion coefficient.
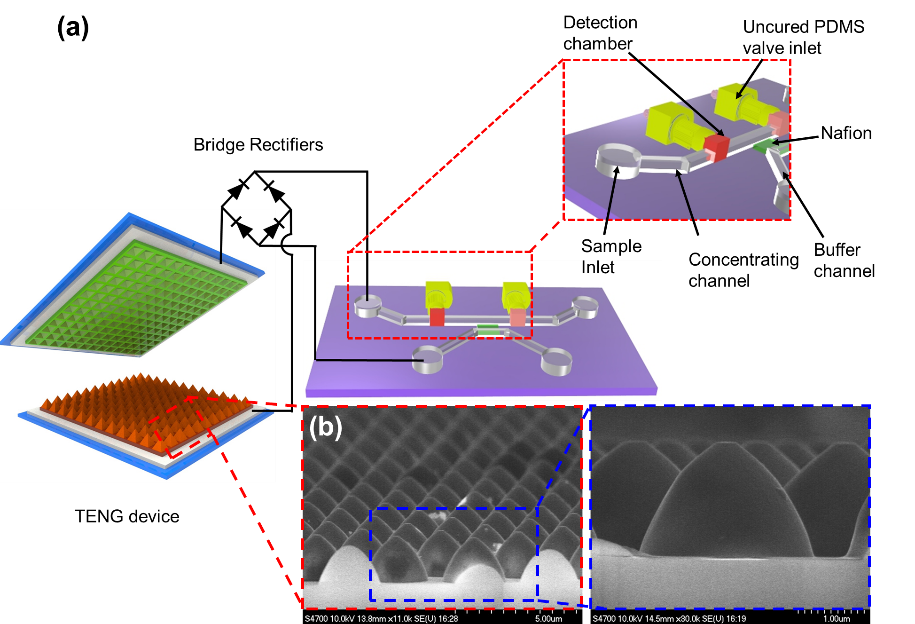
Figure 2. TENG-driven nanofluidic preconcentrating device.
STAY CONNECTED. SUBSCRIBE TO OUR NEWSLETTER.
Add your information below to receive daily updates.




